CHAPTER 1. Clinical Pathology: Clinical Chemistry
Patricia A. Schenck
EVALUATION OF RENAL FUNCTION
I. Blood urea nitrogen (BUN)
A. Decreased glomerular filtration rate (GFR) results in increased BUN
B. Affected by urea production in the liver and the rate of excretion by the kidney
C. Increased dietary protein and gastrointestinal (GI) bleeding both increase BUN
II. Creatinine
A. An elevation indicates that less than 25% of the original functioning renal mass remains
B. A normal serum creatinine concentration does not exclude the possibility of renal disease
C. Young animals have lower serum creatinine concentrations than do older animals
D. Cachexia often causes lower serum creatinine concentrations
III. Serum phosphorus concentration
A. An increase in serum phosphorus is not seen until more than 85% of nephrons are nonfunctional in chronic renal diseases
B. Tubular reabsorption of phosphorus is regulated by parathyroid hormone. Renal secondary hyperparathyroidism tends to keep the serum phosphorus concentration within normal limits by excreting more phosphorus into the urine until renal disease is advanced
C. Serum phosphorus concentrations can be much higher in immature animals because of bone growth
IV. Renal clearance (estimation of GFR)
A. Endogenous creatinine clearance determination
1. Collect all urine for 12 or 24 hours (record volume), and determine serum and urine creatinine concentrations
2. Performed when renal disease is suspected but both BUN and serum creatinine concentrations are normal
B. Exogenous creatinine clearance
1. Creatinine is administered subcutaneously or intravenously (IV); then urine is collected via catheterization three times at 20-minute intervals
2. Better method than endogenous creatinine clearance and approximate inulin clearance in dogs
C. Single-injection methods for estimation of GFR
1. Post-iohexol clearance
a. Give iohexol IV, and collect plasma at 2, 3, and 4 hours postiohexol
b. Plasma clearance is calculated using the area under the plasma concentration vs. time curve
2. Inulin clearance is considered the gold standard for measurement of GFR, but inulin is not easily measured and is not available at commercial laboratories
V. Urine osmolality
A. There is usually a linear relationship between urine osmolality and specific gravity
B. Urine osmolality depends on the number of osmotically active particles present in urine
C. If urine contains a large amount of larger-molecular-weight solutes such as glucose, mannitol, or radiographic contrast agents, the urinary specific gravity will be disproportionately high compared with the osmolality
VI. Fractional excretion of electrolytes
A. Sodium
1. Useful in the differentiation of prerenal and primary renal azotemia
2. In animals with prerenal azotemia and volume depletion, there should be sodium conservation with a very low fractional excretion of sodium
3. In animals with primary renal disease, the fractional excretion of sodium should be higher than normal
B. Potassium
1. May be useful in the evaluation of chronic renal failure patients with hypokalemia to determine whether the kidneys are contributing to the hypokalemia
2. Varies considerably depending on diet
C. Phosphorus
1. May be useful during treatment of chronic renal failure to determine whether dietary or drug therapy is effective with a reduction in fractional excretion of phosphorus
VII. Urinary enzymes
A. γ-glutamyltransferase (GGT) is a membrane-bound enzyme specific for renal tubular damage
B. N-acetyl-β-D-glucosaminidase (NAG)
1. Lysosomal enzyme produced by many tissues but not filtered normally
2. Increases in urinary NAG are specific for renal tubular damage
VIII. Urinalysis
A. Physical properties
1. Color
a. Normally colorless to deep amber in color (if very concentrated). Deep amber color may also be due to bile pigments
b. Red or reddish brown color is due to intact red blood cells (RBCs), hemoglobin, or myoglobin
c. Dark brown to black is most likely due to the conversion of hemoglobin to methemoglobin
d. Yellow-brown to yellow-green is due to bilirubin
e. Green color may be due to Pseudomonas infection or to oxidation of bilirubin to biliverdin
2. Appearance
a. Urine is normally clear in dogs but may be cloudy in about 20%
b. Cloudy urine usually contains increased cells, crystals, mucus, or casts
c. Horse urine is typically cloudy because of mucus
d. Rabbit urine is white and opaque because of the high concentration of calcium carbonate
3. Odor
a. The normal odor of urine is due to volatile fatty acids
b. An ammonia odor is due to release of ammonia by urease-producing bacteria
4. Urine specific gravity (USG) is the weight of urine compared to that of distilled water
a. USG estimated by dipstrip is NOT accurate. USG should be estimated by refractometry. Make sure the refractometer is temperature compensated and has different scales for different species
b. First-morning urine samples typically have the highest urinary concentration
c. Dogs or cats with any detectable dehydration should elaborate maximally concentrated urine (USG >1.040)
B. Chemical examination
1. pH
a. Measurement by pH meter is superior to dipstrip methods
b. Urine pH varies with diet and acid-base balance. Urine pH is usually acidic in carnivores and alkaline in herbivores
c. Causes of acidic urine include meat diets, administration of acidifying agents, metabolic acidosis, respiratory acidosis, or paradoxical aciduria in metabolic alkalosis with potassium and chloride depletion
d. Causes of alkaline urine include plant-based diets, urine that has been allowed to stand open to air at room temperature, postprandial alkaline tide, urinary tract infection (UTI) by urease-positive organisms, contamination of sample with bacteria during or after collection, administration of alkalinizing agents, metabolic alkalosis, respiratory alkalosis, stress induction of respiratory alkalosis (cats), and distal renal tubular acidosis
2. Protein
a. Trace to 1+ protein is normal in urine with high USG
b. Dipstick methods are more sensitive to albumin than globulins
c. False positives occur in very alkaline urine or in urine contaminated with benzylalkonium chloride
d. Renal proteinuria may result from increased glomerular filtration of protein, failure of tubular reabsorption of protein, tubular secretion of protein, protein leakage from damaged tubular cells, or renal parenchymal inflammation
e. Persistent moderate or heavy proteinuria in the absence of urine sediment abnormalities suggests glomerular disease
f. Active sediment with mild to moderate proteinuria suggests inflammatory renal disease or lower urinary tract disease
3. Glucose
a. Normally not present in dog and cat urine
b. Glucose appears in urine if plasma glucose exceeds approximately 180 mg/dL in the dog and 300 mg/dL in the cat
c. Causes of glucosuria include diabetes mellitus (most common), stress or excitement (especially in cats), chronically sick cats in the absence of hyperglycemia, renal tubular disease, administration of glucose-containing fluids, and severe urethral obstruction in some cats
4. Ketones
a. Not normally present in dog and cat urine
b. Inadequate consumption of carbohydrates or impaired utilization of carbohydrates can lead to ketone production
c. Causes of ketonuria include diabetic ketoacidosis (most common), starvation or prolonged fasting, glycogen storage disease, low carbohydrate-high fat diet, and persistent hypoglycemia (decreased insulin induces ketone formation)
5. Bilirubin
a. Only conjugated bilirubin appears in the urine. A small amount of bilirubin may normally be seen in concentrated urine samples from normal male dogs. It is not normally found in cat urine
c. Bilirubin may appear in the urine prior to the observation of hyperbilirubinemia
d. Causes of bilirubinuria include hemolysis, liver disease, extrahepatic biliary obstruction, fever, and starvation
6. Blood
a. Positive earlier than the observation of hematuria
b. Dipstick tests do not differentiate from intact RBCs or hemoglobin
c. Causes of hemoglobinuria from hemolysis include transfusion reaction, immune mediated hemolytic anemia, disseminated intravascular coagulopathy (DIC), splenic torsion, severe hypophosphatemia, heat stroke, zinc toxicity, and phosphofructokinase or pyruvate kinase deficiency
C. Urinary sediment
1. Sediment preparation
a. Perform on fresh urine samples
b. Centrifuge 5 to 10 mL of urine at 1000 to 1500 rpm for 5 minutes. Stain with Sedi-Stain
c. Number of casts is recorded per low-power field, and cells are recorded per high-power field
2. RBCs
a. Occasional RBCs are normal
b. Excessive number of RBCs is called hematuria, but origin cannot be determined
c. Lipid droplets are often confused with RBCs, especially in cats
d. Causes of hematuria include trauma, urolithiasis, neoplasia, UTIs idiopathic feline lower urinary tract disease, chemically induced cystitis, systemic diseases associated with hemorrhage, renal infarct, nephritis, nephrosis, parasites, renal pelvic hematoma, or genital tract contamination
3. White blood cells (WBCs)
a. Occasional WBCs are normal
b. Excessive WBCs in urine sediment is called pyuria and indicates inflammation somewhere in the urinary tract or contamination from the genital tract
c. Clumped WBCs are typically due to infectious organisms
d. Causes of pyuria include urinary tract inflammation or genital tract contamination
4. Epithelial cells
a. Squamous epithelial cells
(1) Large, polygonal cells with small, round nuclei
(2) Common in voided or catheterized samples
b. Transitional epithelial cells
(1) A small number is normal
(2) Increased in infection, irritation, or neoplasia
(3) Clumps or “rafts” are most common in neoplasia but may occur with inflammation
c. Renal epithelial cells
(1) Small epithelial cells from the renal tubules or pelvis
(2) Appearance in urine is never normal and is observed in patients with ischemic, nephrotoxic, or degenerative renal disease
5. Casts are cylindrical molds of the renal tubules composed of aggregated protein or cells
a. Hyaline
(1) Pure protein precipitates of Tamm-Horsfall mucoprotein
(2) Dissolve rapidly in dilute or alkaline urine
(3) Have the least pathologic significance and may form transiently with fever, exercise, or passive congestion to the kidney
b. Cellular casts
(1) White cell casts suggest pyelonephritis but may also be caused by interstitial nephritis, nephrosis, or glomerulonephritis
(2) Red cell casts are fragile and rarely found. They may be noted in acute glomerulonephritis, renal trauma, or after violent exercise
(3) Hemoglobin casts are casts where the hemoglobin color is retained in the cast
(4) Renal epithelial casts occur with severe tubular injury and suggest acute tubular necrosis or pyelonephritis (Figure 1-1)
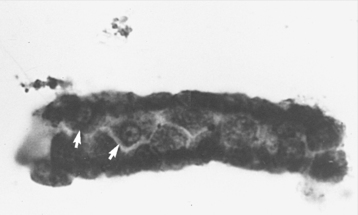 |
| Figure 1-1 Photomicrograph of an epithelial cell cast in urine. Small renal epithelial cells can be identified in this case (white arrows). (Courtesy Nancy Facklam; from Ettinger SJ, Feldman EC. Textbook of Veterinary Internal Medicine, 6th ed. St Louis, 2005, Saunders.) |
(5) Renal fragments are a variant of epithelial casts where portions of the renal tubules slough into urine. Their appearance suggests severe renal injury
(6) Mixed casts contain multiple cell types
c. Granular casts (Figure 1-2)
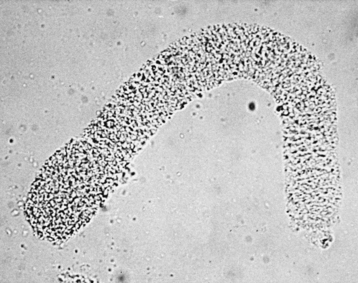 |
| Figure 1-2 Photomicrograph of a finely granular case in urine. (From Ettinger SJ, Feldman EC. Textbook of Veterinary Internal Medicine, 6th ed. St Louis, 2005, Saunders.) |
(1) Represent the degeneration of cells or precipitation of filtered plasma proteins
(2) Fatty casts are a type of granular cast that may be seen in nephrotic syndrome or diabetes mellitus
e. Broad casts are wide casts that form in collecting ducts or dilated distal nephron. They suggest severe intrarenal stasis and tubular obstruction
6. Organisms
a. Normal urine is sterile
b. Large numbers of bacteria present (in association with pyuria) in urine collected by catheterization or cystocentesis strongly suggest the presence of UTI
c. The presence of bacteria without pyuria should arouse suspicion for bacterial contamination. However, dogs with hyperadrenocorticism, diabetes mellitus, or immunosuppression and cats with chronic renal disease can have bacteriuria with pyuria
d. The absence of bacteria does not rule out UTI
e. Yeast and fungal hyphae in sediment are usually contaminants
7. Crystals
a. Crystals are often an artifact of storage time and refrigeration
b. Struvite crystals are found in alkaline urine and may be found in normal animals or in those with struvite urinary stones
c. Calcium phosphate crystals are found in alkaline urine
d. Calcium carbonate crystals are found in alkaline urine
e. Amorphous phosphate crystals are found in alkaline urine
f. Ammonium biurate crystals are found in alkaline urine
g. Uric acid crystals are found in acidic urine and are associated with the Dalmatian breed
h. Urate crystals are associated with liver disease and portosystemic shunt
i. Calcium oxalate crystals are found in acidic urine
j. Cystine crystals are found in acidic urine and are associated with cystinuria
k. Bilirubin crystals may be found normally in concentrated dog urine
l. Oxalate monohydrate (“hippurate-like”) crystals are found in acute renal failure owing to ethylene glycol ingestion
8. Miscellaneous
a. Sperm is common in urine from intact males
b. Amorphous debris may be seen in those with acute intrinsic renal failure
c. Mucous threads or fibrin strands may be seen in association with lower urinary tract or genital inflammation
d. Parasite ova from Dioctophyma renale or Capillaria plica are rarely seen
e. Lipid droplets are associated with cellular degeneration
f. Foreign material may be present, especially in voided samples
g. Precipitates of urine stain may look like urinary crystals
FLUIDS AND ACID-BASE METABOLISM
I. Dehydration
A. Status
1. Total body water is about 60% of body weight; about half is extracellular and half is intracellular
2. Very mild dehydration occurs with water loss of 1% to 4% of body weight. Clinical signs are not detectable
3. Mild dehydration occurs with water loss of 5% to 6% of body weight. Clinical signs include dry mucous membranes, slight loss of skin turgor, injected conjunctiva, and inelasticity of skin
4. Moderate dehydration occurs with water loss of 7% to 9% of body weight. Clinical signs include loss of skin turgor with slow return, prolonged capillary refill time (2-3 seconds), enophthalmos
5. Severe dehydration occurs with water loss of 10% to 12% of body weight. Clinical signs include extreme loss of skin turgor, peripheral vasoconstriction, cold extremities, and prolonged capillary refill time (>3 seconds)
6. Very severe dehydration occurs with water loss of 13% to 15% of body weight; clinical signs include vascular collapse, renal failure, and death
B. Isotonic dehydration occurs with equal losses of water and solute
1. Sodium and chloride concentrations are not affected
2. Increased packed cell volume (PCV) with increased plasma proteins
3. Occurs in some cases of diarrhea and renal disease
1. Concentration of sodium and chloride increases
2. PCV increases, with increased plasma proteins
3. Occurs most commonly in diabetes insipidus
4. Species that produce hypotonic sweat (cattle) or little sweat (dogs, cats) develop hypertonic dehydration with heat stress
D. Hypotonic dehydration occurs when more solute than water is lost
1. Concentrations of sodium and chloride decrease
2. This results in a contraction of the extracellular fluid (ECF) volume with expansion of intracellular fluid (ICF) volume to restore osmotic equilibrium
3. Most common type of dehydration, where the solute loss induces a secondary loss of water
4. Hypotonic dehydration from heat stress occurs in species that produce hypertonic sweat (horses)
II. Acid-base metabolism
A. Henderson-Hasselbach equation
1. pH = pK a + log [A −]/[HA]
2. The carbonic acid-bicarbonate system is usually used: pH = pK a + log [HCO 3−]/[H 2CO 3]
3. pH = 6.1 + log[HCO 3−]/0.03(P co2)
B. To characterize acid-base disorders, blood pH, HCO 3, and P co2 are measured (Table 1-1)
| Primary events are indicated by double arrows. | ||||
| pH | [H+] | [HCO 3] | Pco 2 | |
|---|---|---|---|---|
| Metabolic alkalosis | ↑ | ↓ | ↑↑ | ↑ |
| Metabolic acidosis | ↓ | ↑ | ↓↓ | ↓ |
| Respiratory acidosis | ↓ | ↑ | ↑ | ↑↑ |
| Respiratory alkalosis | ↑ | ↓ | ↓ | ↓↓ |
| GI loss (vomiting, diarrhea) | ||||
1. A decrease in pH is acidosis; an increase is alkalosis
2. A decrease in HCO 3 is a metabolic acidosis; an increase is a metabolic alkalosis
3. A decrease in P co2 is a respiratory alkalosis; an increase is a respiratory acidosis
4. If HCO 3 measurement is unavailable, total CO 2 from a chemistry profile can be used as an estimate. Total CO 2 is about 1 to 2 mmol/L greater than the HCO 3 concentration
C. Metabolic disorders
1. Characterized by changes in HCO 3
2. Compensation is via rapid changes in ventilation to alter P co2
D. Respiratory disorders
1. Characterized by changes in P co2
2. Compensation is via a change in urinary acidification to alter HCO 3. This process is slower than compensation in ventilation
E. Simple acid-base disorders occur when there is a primary change, but no compensation has taken place
F. Compensated acid-base disorders occur when primary changes are present, along with evidence of a compensatory change in the complementary system. The pH rarely returns to normal with compensation
G. Combined acid-base disorders occur when there are changes in the same direction in both systems
H. Metabolic acidosis
1. Primary change is decreased HCO 3
2. P co2 will decrease in compensation
I. Metabolic alkalosis
1. Primary change is increased HCO 3
2. P co2 will increase in compensation
J. Respiratory acidosis
1. Primary change is increased P co2
2. HCO 3 will increase in compensation
3. There is a larger compensation in chronic respiratory acidosis compared with an acute event
K. Respiratory alkalosis
1. Primary change is decreased
2. P co2
3. HCO 3 will decrease in compensation
4. There is a larger compensation in chronic respiratory alkalosis compared with an acute event
L. Base excess and base deficit
1. Calculated from blood gas parameters by the blood gas analyzer. This calculation is based on human relationships and is probably valid for dogs and cats. This calculation might not be valid for other species
2. Increased values reflect a base excess corresponding to metabolic alkalosis
3. Decreased values reflect a base deficit, corresponding to metabolic acidosis
M. Anion gap
1. Anion gap = (Na + K) – (Cl + HCO 3); the objective is to estimate changes in the unmeasured anions and cations without having to measure them
a. Unmeasured anions include sulfate, lactate, phosphate, pyruvate, albumin, and ketoacids
b. Unmeasured cations include primarily calcium and magnesium
2. If the anion gap increases, then unmeasured anions have increased. If the anion gap decreases, then unmeasured cations have increased
III. Osmolality
A. Osmolality is the concentration or number of osmotically active particles in an aqueous solution
B. Osmolal gap is the difference between the actual measured serum osmolality and the calculated estimate of osmolality
2. The osmolal gap increases when there is an increase in any osmotically active particles that are not included in the calculated equation
3. The osmolal gap will increase whenever the anion gap is increased
4. Used commonly in cases of ethylene glycol toxicity
a. Ethylene glycol is a small osmotically active particle
b. The osmolal gap correlates well with the concentration of ethylene glycol in serum
ELECTROLYTE METABOLISM
I. Sodium
A. Roles
1. Principal cation in ECF
2. Important in movement of fluids across epithelial surfaces
B. Hyponatremia
1. Pseudohyponatremia
a. Occurs with hyperlipidemia or hyperproteinemia
b. Plasma sample is diluted by the excess lipid or protein and thus the measured sodium concentration is falsely lowered
2. Hyperosmolal, hypervolemic conditions include hyperglycemia and mannitol administration
3. Hypoosmolal hypervolemic conditions
a. Occurs when there is excess water retention with dilution of the plasma
b. Causes include nephrotic syndrome, chronic liver disease, chronic renal failure, and congestive heart failure
4. Hypoosmolal euvolemic conditions include hypotonic fluid infusion, antidiuretic hormone (ADH) administration, inappropriate secretion of ADH, and psychogenic polydipsia
5. Hypoosmolal hypovolemic conditions include the following:
a. Dietary deficiency of sodium
b. GI loss from vomiting or diarrhea
c. Third-space syndrome (GI obstruction, peritonitis, uroabdomen, ascites)
d. Urinary loss from hypoadrenocorticism, nonoliguric acute renal failure, diuretics, and Fanconi syndrome
e. Cutaneous losses (burns)
C. Hypernatremia
1. Pure water deficits occur in dietary deficiency, central or nephrogenic diabetes insipidus, primary hypodipsia, heat stress, and fever
2. Hypotonic fluid loss occurs with the following:
a. GI loss owing to vomiting or diarrhea
b. Third-space syndrome (peritonitis, ascites)
c. Urinary loss from osmotic diuretics (mannitol, diabetes mellitus), chronic renal failure, nonoliguric acute renal failure, postobstructive nephropathy
d. Cutaneous loss (burns)
3. Solute gain occurs with salt poisoning, hypertonic fluid administration, hyperadrenocorticism, hyperaldosteronism
II. Chloride
A. Roles
1. Principal anion in ECF
2. Chloride usually accompanies sodium to maintain neutrality
3. Normal fractional excretion is less than 1% but may be elevated in large animals fed a diet higher in chlorine
B. The same conditions causing hypernatremia and hyponatremia also cause hyperchloremia and hypochloremia
III. Potassium
A. Roles
1. Principal cation of the ICF
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


