9 Zoonoses
Overview
Key Points for Clinicians and Public Health Professionals
Public Health Professionals
Human Health Clinicians
Veterinary Clinicians
INFECTIOUS DISEASES IN HUMANS AND OTHER ANIMALS: FROM “US VERSUS THEM” TO “SHARED RISK”
Despite the great deal of attention that has been focused on emerging infectious zoonotic diseases, including severe acute respiratory syndrome (SARS), West Nile virus, monkeypox, and avian influenza, there has been less discussion and effort targeted at the environmental “drivers” of such diseases. One possible reason is that the traditional approach of the human health community to zoonotic disease has been an “us versus them” approach. The problem is viewed as an infectious animal reservoir that then poses an infectious risk to humans—either through direct contact with infected animals and their excretions, meat, milk, or other tissues, or via a vector transmission bringing the pathogen from the animal population into human hosts. The control of such “us versus them” diseases has traditionally involved measures such as control of the animal reservoir (through culling, quarantine, or vaccination) or vector control (through pesticides and personal protection). For many zoonotic diseases, however, such approaches are limited because the ultimate causes of infection in the animals may not be addressed sufficiently. For example, Nipah virus emerged as a deadly pathogen in Malaysia when pig farms were built close to forest areas frequented by fruit bats (Figure 9-1 and Color Plate 9-1). These fruit bats, natural hosts for Nipah and other henipaviruses, had sufficient contact with the pig farms to allow the virus pathogen to “spill over” from the wildlife reservoir into the domestic pig population, causing mortality for pigs and humans (and cats) in contact with them.1
Therefore, for many infectious diseases that cross between animals and humans, it is advisable for human health professionals to move beyond an “us versus them” view of animals and infections and to instead join veterinarians and public health professionals to examine the environmental forces driving disease emergence that constitute a “shared risk” of infection for both humans and animals.2 Figure 9-2 outlines these relationships.
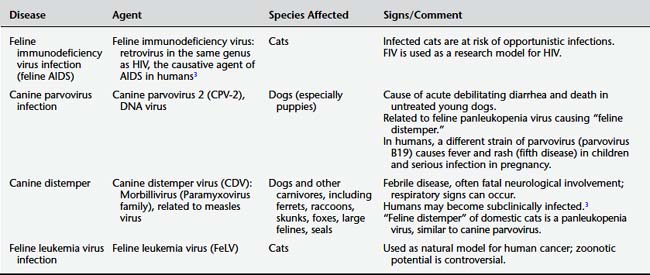
NON-ZOONOSES (FOR NOW)
Experience has shown that this situation may change as organisms continue to adapt to new environments and acquire mutations that allow them to cross species barriers. Nonetheless, human health clinicians should (1) be aware of animal diseases that, based on current knowledge, do not appear to cause disease in humans and (2) be able to reassure patients who express such concerns. Similarly, clinicians can correct misinformation regarding species-specific human diseases that patients may believe come from animal contact. For example, human pinworm infection is not a zoonotic disease. Table 9-1 lists some of these non-zoonoses. As this list shows, many of these agents, while currently not considered zoonotic to any significant degree, bear some relation to human pathogens.
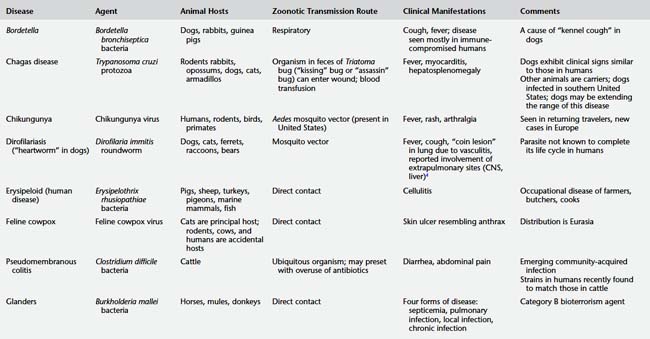
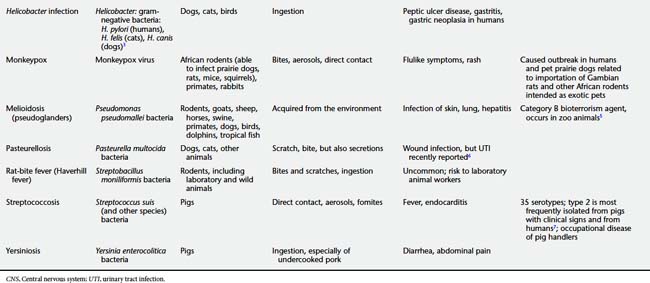
DISEASES TO WATCH
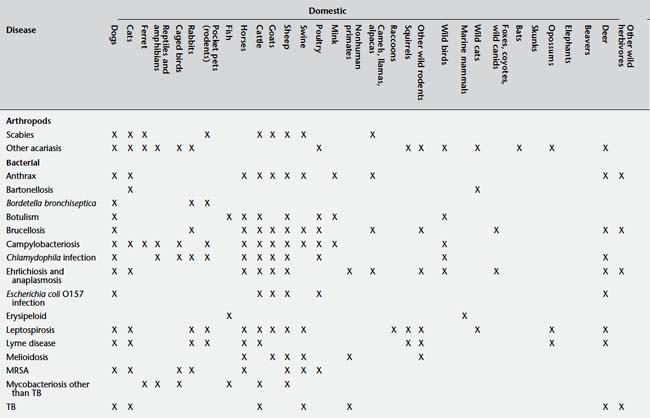
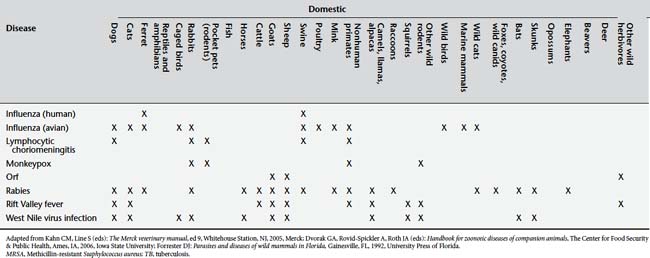
Table 9-2 presents a number of pathogens and diseases for which clinicians and public health professionals should maintain an awareness of possibly increasing zoonotic disease risk. Table 9-3 lists a number of currently recognized zoonotic pathogens and some of the species for which the pathogen has been reported.
1. Daszak P., Cunningham A.A., Hyatt A.D. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78(2):103.
2. Rabinowitz P.M., Odofin L., Dein F.J. From “us vs. them” to “shared risk”: can animals help link environmental factors to human health? EcoHealth. 2008;5(2):224.
3. Barr S.C., Bowman D.D. 5-minute veterinary consult clinical companion canine and feline infectious disease and parasitology. Ames, IA: Wiley-Blackwell, 2006.
4. Theis J.H. Public health aspects of dirofilariasis in the United States. Vet Parasitol.. 2005;133(2–3):157.
5. Kahn C.M., Line S., editors. The Merck veterinary manual, 9th ed., Whitehouse Station, NJ: Merck, 2005.
6. Liu W., Chemaly R.F., Tuohy M.J., et al. Pasteurella multocida urinary tract infection with molecular evidence of zoonotic transmission. Clin Infect Dis.. 2003;36(4):E58.
7. Dvorak G.A., Rovid-Spickler A., Roth J.A., editors. Handbook for zoonotic diseases of companion animals. The Center for Food Security & Public Health; Iowa State University: Ames, IA, 2006, 234.
Anthrax
Cutaneous anthrax (ICD-10 A22.0), Pulmonary anthrax (A22.1), Gastrointestinal anthrax (A22.2)
Other names in humans: wool sorter’s disease, charbon, malignant carbuncle, Siberian ulcer
Other names in animals: splenic fever, Milzbrand
Anthrax is a fatal disease of herbivores. Most human cases result from direct contact with sick or dead animals or contaminated animal products. The causative agent, Bacillus anthracis, is a Centers for Disease Control and Prevention (CDC) Category A bioterrorism agent, and the anthrax-tainted letters mailed in the United States in 2001 were a reminder of its potential for deliberate release. In the United States, anthrax outbreaks in wildlife and livestock occur annually but human cases are rare.1 Veterinary and human health care professionals need to recognize the clinical signs of disease and report suspected cases to public health authorities.
Key Points for Clinicians and Public Health Professionals
Public Health Professionals
Human Health Clinicians
Veterinary Clinicians
Agent
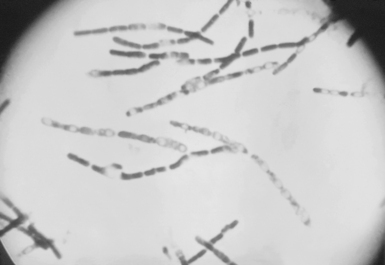
The bacterium B. anthracis is a spore-forming, nonmotile, gram-positive bacillus 3 to 5 microns long. When the vegetative form is exposed to air, it sporulates to form infectious spores. The spores may survive for decades in soil (Figure 9-3).
Geographical Occurrence
National disease control programs have reduced the global incidence of anthrax. It remains common in some Mediterranean countries, localized areas of Canada and the United States, parts of Central and South America, central Asia, parts of sub-Saharan Africa, and western China.9 An epizootic among cattle in South Dakota in 2000 resulted in 157 cattle deaths and one human case of cutaneous anthrax.10 Human anthrax resulting from exposure to infected livestock remains rare in the United States but occurs more commonly in less-developed countries. In many regions, the true incidence is not known because many cases in animals and humans probably go unreported.
Groups at Risk
Anthrax can be an occupational disease of workers who process carcasses and hides of infected animals, including farmers, abattoir workers, butchers, and workers in factories that process hides. Veterinarians who handle sick animals are also at risk, as are laboratory workers who routinely work with B. anthracis. Cases of both inhalation and cutaneous anthrax have developed in travelers and drum makers who have bought drums or hides originating from infected animals. In 2007, two cases of cutaneous anthrax in Connecticut were tied to importation of infected goat hides for drum-making (Figure 9-4).2

Figure 9-4 Bacillus anthracis–contaminated drum head made from goat hide from Guinea; Connecticut, 2007.
(From Centers for Disease Control and Prevention: Cutaneous anthrax associated with drum making using goat hides from West Africa—Connecticut, 2007, MMWR Morbid Mortal Weekly Rep 57(23):628, 2008.)
Hosts, Reservoir Species, Vectors
The reservoir for anthrax is the environment, where the spores can survive for years in alkaline calcium-rich soil. Anthrax is principally a disease of livestock, including cattle, sheep, goats, and camels. Wild ruminants such as antelope and bison can also be infected and pose a risk to livestock.11 However, all mammals are susceptible,12 and horses, pigs, dogs, cats, and humans can be incidentally infected. Because of their higher body temperatures birds are normally resistant, but ostriches are susceptible. In some settings, biting flies may serve as vectors for anthrax transmission.9
In the 1979 accidental release of aerosolized anthrax in Sverdslovsk, Russia, cattle and sheep died as far as 50 km downwind from the release site, while human cases of inhalation anthrax occurred only up to 4 km downwind from the release.13 The fact that animals became sickened over a wider geographical area than did humans may reflect their increased susceptibility and increased exposure risk, making animal cases sentinels for human risk.
Mode of Transmission and Life Cycle
Infected animals release vegetative bacteria into the environment. As the bacteria are exposed to air, they sporulate and the spores can survive for years. Ruminant animals grazing on areas contaminated by spores can ingest the spores and become infected. This can lead to further contamination of the environment and additional animal cases (Figure 9-5). Biting flies appear to play a role in large outbreaks by facilitating animal-animal transmission, sometimes over significant distances (5 to 15 km). Direct animal-animal transmission among herbivores is considered insignificant,9 but if carnivores eat the flesh of infected animals, they can become infected.
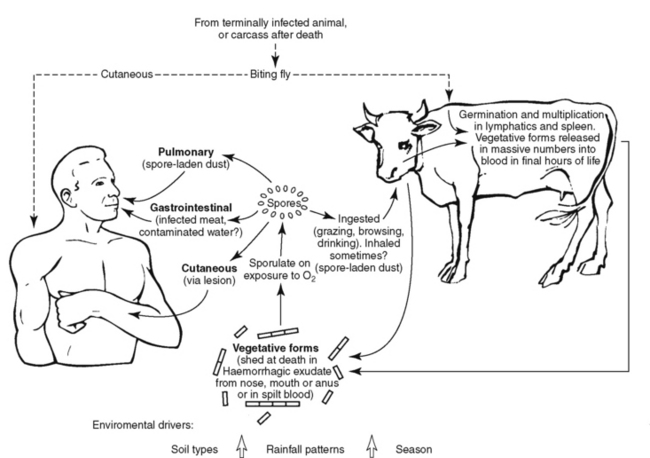
Figure 9-5 Cycle of anthrax infection.
(Modified from Guidelines for the surveillance and control of anthrax in humans and animals, ed 4, Geneva, 2008, World Health Organization. Available at http://www.who.int/csr/resources/publications/anthrax/whoemczdi986text.pdf.)
Most human infection occurs through direct contact with animals or animal products. Such transmission is more likely when there are breaks in the skin and leads to the cutaneous form of the disease (Figure 9-6).

Figure 9-6 Cutaneous anthrax in a child.
(From Roche KJ, Chang MW, Lazarus H: Images in clinical medicine: cutaneous anthrax infection, N Engl J Med 345:1611, 2001.)
Environmental Risk Factors
The persistence of spores in the environment depends on a number of factors, including temperature, humidity, soil pH, calcium and other cations in soil, and the abundance of soil bacteria that could break down spores.14 Outbreaks in wildlife species have been linked to climate factors such as hot, dry weather following spring flooding.11 Heavy rainfall may increase the population of biting flies that can amplify animal outbreaks and may lead to spores being brought to the soil surface.9,12
Anthrax spores can contaminate indoor environments. One of the recent cases in Connecticut tied to imported drum hides was the child of the drum maker, who became infected through contamination of the household environment.2
Disease in Humans
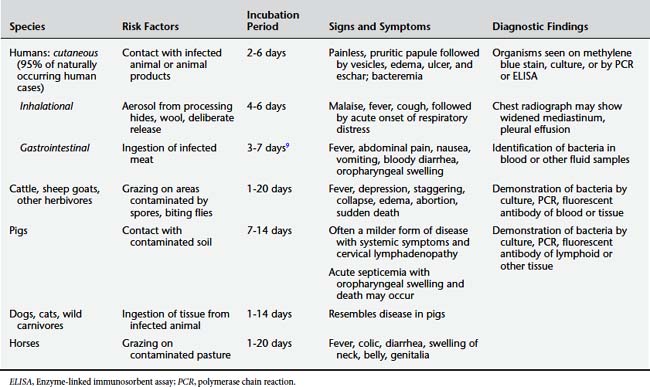
There are three main forms of the disease: cutaneous, inhalational, and gastrointestinal. In naturally occurring human disease, more than 95% of cases are the cutaneous form. Inhalational anthrax is the next most common form. Gastrointestinal anthrax has never been reported in the United States. Table 9-4 shows the comparative clinical presentations of anthrax in humans and animals.
Cutaneous Anthrax
Cutaneous anthrax begins 1 to 7 days after inoculation with a small, painless, pruritic papule that is often asymptomatic and does not lead to an infected individual seeking medical care. Over a period of days, vesicles develop with surrounding edema, which then rupture to form an ulcer covered by a black eschar. A significant degree of edema develops around the eschar and can be severe. Most cutaneous lesions are on the hands and arms. If the head and neck are involved, breathing may be compromised by the swelling. Bacteremia can develop, leading to systemic complications. Without antibiotic treatment the mortality rate is approximately 20%.15 Figure 9-6 shows cutaneous anthrax in a child.
Inhalational Anthrax
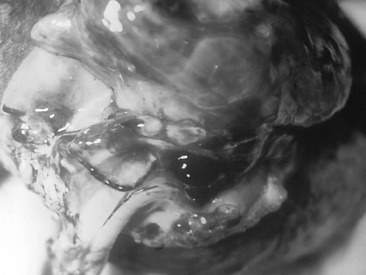
Inhalational anthrax often begins with nonspecific malaise, mild fever, and nonproductive cough 1 to 6 days after exposure. After several days, a second phase of the disease begins abruptly with high fever, dyspnea, cyanosis, and stridor. A hemorrhagic lymphadenitis can develop with mediastinal widening (Figure 9-7). Without treatment, respiratory decompensation soon occurs. In up to half of cases, anthrax meningitis may also be present, with meningeal signs and altered consciousness.15 Mortality rate is high even with antibiotic treatment.
Gastrointestinal Anthrax
Gastrointestinal anthrax is characterized by fever, abdominal pain, and bloody diarrhea that develop 2 to 5 days after ingestion of contaminated meat (to date, it has not been reported in the United States). Oropharyngeal involvement can produce swelling with respiratory compromise. The mortality rate can be 25% to 75%.16
Disease in Animals
Dogs, cats, nonhuman primates, and wild carnivores can develop disease resembling that in pigs (Figure 9-8).15
Diagnosis
Diagnosis in Humans
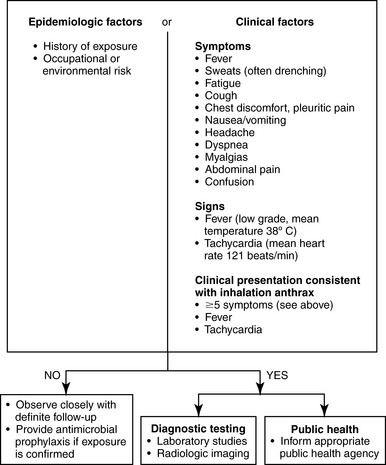
Inhalational anthrax can present with nonspecific symptoms and may be confused with other causes of pneumonitis, including community-acquired pneumonia and influenza. In the second, severe stage of illness, possible considerations include aortic dissection, pneumonic plague, and hantavirus pulmonary syndrome. A screening protocol for inhalation anthrax has been proposed by a consensus report (Figure 9-9). This protocol is based on history of exposure and the presence of clinical signs.
The differential diagnosis of oropharyngeal anthrax includes streptococcal pharyngitis and Ludwig’s angina.9
The laboratory diagnosis of anthrax involves identification of the capsulated organism in blood or tissues using methylene blue (M’Fadyean)-stained smears or through bacterial culture of blood or other specimens.16 Rapid tests that are increasingly available include polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), and immunohistochemical staining. Diagnostic testing for suspected inhalation anthrax in humans should include chest radiography and/or chest computed tomographic (CT) scanning to look for mediastinal widening.1
Diagnosis in Animals
Diagnostic testing can be performed on a swab of blood that is allowed to air-dry, resulting in sporulation of the bacteria and death of other bacteria and contaminants. In pigs, lymph tissue should be sent for studies. Bacterial culture, PCR, and fluorescent antibody stains can demonstrate the organism in blood and tissues.17
Treatment
Treatment in Humans
Treatment of anthrax in humans involves treatment with antibiotics as soon as the disease is suspected or as PEP. Table 9-5 shows the recommended initial treatment regimens. Although quinolones or doxycycline are first-line agents, if the infecting strain is found to be susceptible to penicillin, penicillin can be substituted.
Table 9-5 Initial Treatment of Anthrax in Human Beings and Animals
| Species | Primary Treatment | Alternative Treatment |
|---|---|---|
| Humans: | ||
| Cutaneous | Ciprofloxacin 500 mg PO bid or levofloxacin 500 mg IV/PO bid × 60 days Children <50 kg: ciprofloxacin 20-30 mg/kg/day divided q12h PO (maximum 1 gm/day) × 60 days or levofloxacin 8 mg/kg PO q12h × 60 days | Doxycycline 100 mg PO bid × 60 days Children >8 yr and >45 kg: doxycycline 100 mg PO bid × 60 days <8 yr: doxycycline 2.2 mg/kg PO bid × 60 days18 |
| Inhalational and gastrointestinal | Adult: Ciprofloxacin 400 mg IV q12h or levofloxacin 500 mg IV q24h PLUS clindamycin 900 mg IV q8h and/or rifampin 300 mg IV q12h; treatment duration 60 days; switch to PO when able | Children: ciprofloxacin 10 mg/kg IV q12h or 15 mg/kg PO q12h or doxycycline (>8 yr and >45 kg) 100 mg IV q12h, PLUS clindamycin 7.5 mg/kg IV q6h and/or rifampin 20 mg/kg (maximum 600 mg) IV q24h; treatment duration 60 days19 |
| Postexposure prophylaxis | Ciprofloxacin 500 mg PO bid or levofloxacin 500 mg PO q24h × 60 days Children: ciprofloxacin 20-30 mg/kg/day divided q12h × 60 days | Doxycycline 100 mg PO bid × 60 days Children >8 yr and >45 kg: doxycycline 100 mg PO bid <8 yr: doxycycline 2.2 mg/kg PO bid × 60 days |
| Cow, sheep, goat, horse | Penicillin | Oxytetracycline |
| Dog19 | Oxytetracycline 5 mg/kg IV q24h Potassium penicillin G at 20,000 U/kg IV q8h | Enrofloxacin 5 mg/kg q24h |
ADDITIONAL RESOURCES
CDC Advisory Committee on Immunization Practices Recommendations for Use of Anthrax Vaccine
Antimicrobial Prophylaxis
1. Shadomy T.L., Smith S.V. Zoonosis update: Anthrax. J Am Vet Med Assoc.. 2008;233(1):63.
2. Centers for Disease Control and Prevention (CDC). Cutaneous anthrax associated with drum making using goat hides from west Africa—Connecticut, 2007. MMWR Morb Mortal Wkly Rep.. 2008;57(23):628.
3. Meehan P.J., Rosenstein N.E., Gillen M., et al. Responding to detection of aerosolized Bacillus anthracis by autonomous detection systems in the workplace. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr53e430-2a1.htm. Accessed August 11, 2008
4. National Institute for Occupational Safety and Health. NIOSH respiratory diseases research program: evidence package for the National Academies’ Review 2006–2007: 6.2 Anthrax. http://www.cdc.gov/niosh/nas/RDRP/ch6.2.htm. Accessed August 11, 2008
5. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep.. 2000;49(RR15):1.
6. Notice to readers. Use of anthrax vaccine in response to terrorism: supplemental recommendations of the Advisory Committee on Immunization Practices. MMWR.. 2002;51:1024. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5145a4.htm
7. Military Vaccine Agency. Anthrax vaccine immunization program. http://www.anthrax.osd.mil;. Accessed August 11, 2008
8. United States Department of Agriculture. Animal and Plant Health Inspection Service: APHIS factsheet: anthrax—general information and vaccination. http://www.aphis.usda.gov/publications/animal_health/content/printable_version/fs_ahanthravac.pdf. Accessed August 11, 2008
9. World Health Organization. Anthrax in humans and animals. http://www.who.int/csr/resources/publications/anthrax/whoemczdi986text.pdf.
10. Centers for Disease Control and Prevention. Human anthrax associated with an epizootic among livestock—North Dakota, 2000. MMWR Morb Mortal Wkly Rep.. 2001;50(32):677.
11. Nishi J.S., Dragon D.C., Elkin B.T., et al. Emergency response planning for anthrax outbreaks in bison herds of northern Canada. Ann N Y Acad Sci.. 2002;969:245.
12. Hugh-Jones M.E., de Vos V. Anthrax in wildlife. Rev Sci Tech Off Int Epiz.. 2002;21:359.
13. Meselson M., Guillemin J., Hugh-Jones M., et al. The Sverdlovsk anthrax outbreak of 1979. Science.. 1994;266(5188):1202.
14. Acha P.N., Szyfres B. Zoonoses and communicable diseases common to man and animals: vol. 1: bacterioses and mycoses, 3rd ed. Washington, DC: Pan American Health Organization, 2001.
15. Swartz M.N. Recognition and management of anthrax—an update. N Engl J Med.. 2001;345(22):1621.
16. Kahn C.M., Line S., editors. The Merck veterinary manual, 9th ed., Whitehouse Station, NJ: Merck, 2005.
17. US Food and Drug Administration, Center for Biologics Evaluation and Research. Anthrax. http://www.fda.gov/cber/vaccine/anthrax.htm. Accessed August 11, 2008
18. Gilbert D.N., Moellering R.C., Ellopoulos G.M., et al. Sanford guide to antimicrobial therapy 2009, 39th ed. Sperryville, VA: Antimicrobial Therapy, 2009.
19. Langston C. Postexposure management and treatment of anthrax in dogs—executive councils of the American Academy of Veterinary Pharmacology and Therapeutics and the American College of Veterinary Clinical Pharmacology. http://www.aapsj.org/view.asp?art=aapsj070227. Accessed August 11, 2008
Bartonella Infections
Bartonella infection (ICD-10 A28.1)
Other names in animals: bartonellosis
Human Health Clinicians
Veterinary Clinicians
Agent
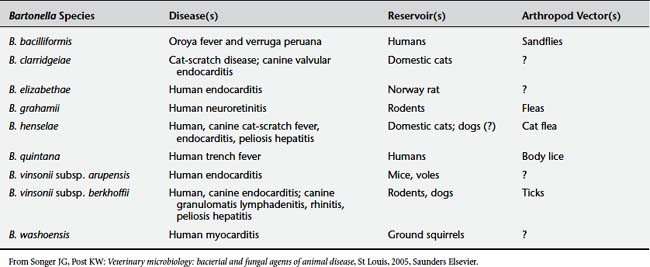
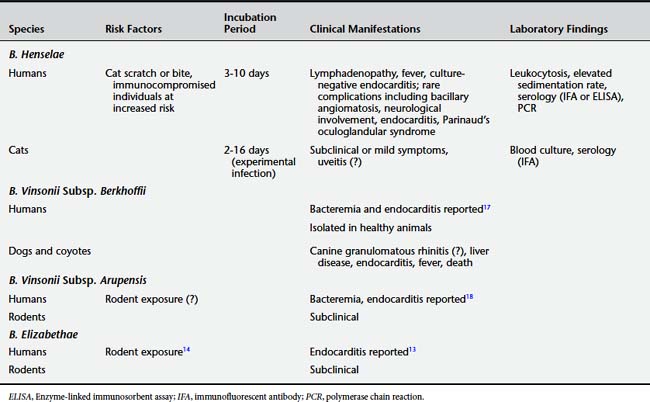
B. henselae is a gram-negative bacillus in the family Bartonellaceae. This family shares some features with rickettsial organisms but has been removed from the order Rickettsiales. The genus Bartonella includes at least 20 species, five of which (B. henselae, B. quintana, B. bacilliformis, B. vinsonii subspecies arupensis, and B. elizabethae) are recognized human pathogens (Table 9-6).2 At present, no animal reservoirs of B. quintana or B. bacilliformis have been identified. B. henselae, B. vinsonii, and B. elizabethae are known to infect both human and animal hosts.3
Groups at Risk
CSD can be an occupational disease of veterinarians and others providing care to cats. Studies of zookeepers have revealed seroprevalence rates for past Bartonella infection as high as 65%,4 while a convenience survey of veterinarians and veterinary workers found Bartonella species seroprevalence of only 7%.
Hosts, Reservoir Species, Vectors
Bartonella species have been found in a wide range of subclinically infected mammals, including rodents, rabbits, deer, elk, bighorn sheep, cattle, foxes, dogs, and coyotes.5 Domestic cats are considered the principal reservoir for B. henselae.6 Seroprevalence studies have shown rates of antibody positivity in cats in the range of 40%.7 However, infection in cats is considered either subclinical or subtle, even in the setting of chronic bacteremia. B. henselae has also been isolated from cat fleas, dog fleas, and a number of other vectors.8 Dogs may also be infected with B. henselae. Recent data show that ticks may play a role in transmission to humans.9
B. quintana at present is known to have only a human reservoir and is spread by the human body louse. B. vinsonii and B. elizabethae have been found in asymptomatic rodent reservoirs, including rural mice (B. vinsonii)10 and urban rats (B. elizabethae).11 Infection to humans may occur through vectors or direct contact.
Mode of Transmission and Life Cycle
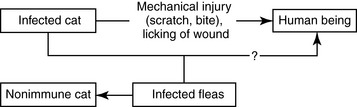
Despite the fact that B. henselae can occur in vectors, transmission to humans is thought to be mainly mechanical through a scratch or bite or licking of an open wound or rubbing the eyes with contaminated hands (Figure 9-10). The role of fleas in transmission is not well understood.
Disease in Humans
Cat scratch infection produces an inoculation at the point of injury, with inflammation of nearby lymph nodes several weeks later (Color Plate 9-2). The lymph swelling often is self-limited over a period of months in immunocompetent hosts (Figure 9-11). In up to one sixth of cases the lymph nodes suppurate. Other symptoms can include malaise, fatigue, fever, and rash.3
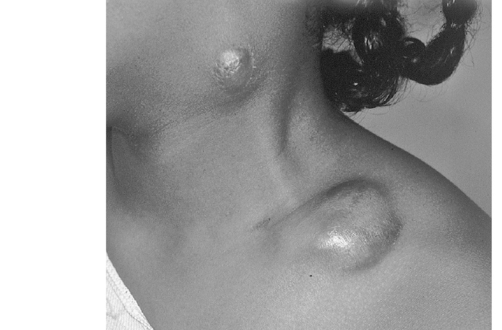
Figure 9-11 Papular lesion and enlarged lymph nodes in a person with cat scratch disease.
(From Long SS, Pickering LK, Prober CG (eds): Principles and practice of pediatric infectious diseases, ed 3, Philadelphia, 2008, Saunders Elsevier.)
Atypical presentations of B. henselae infection include Parinaud’s oculoglandular syndrome (granulomatous conjunctivitis accompanied by pretragal lymphadenopathy).3 Even in immunocompetent patients, serious complications of B. henselae infection can occur, such as central nervous system (CNS) involvement (including encephalopathy and myelitis)12 and hepatic abscesses. In many but not all cases, CSD precedes the development of more serious complications. In the elderly, B. henselae endocarditis may be found more frequently (a common cause of culture-negative endocarditis), whereas CSD is less frequent than in younger individuals.12
In immunocompromised individuals, complications of infection can include bacillary angiomatosis (Color Plate 9-3). Peliosis hepatis, a condition characterized by fever, chills, hepatosplenomegaly, and gastrointestinal symptoms, can develop in immunocompromised patients. Like B. henselae, B. quintana causes a number of conditions, including trench fever, endocarditis, bacillary angiomatosis, and peliosis hepatitis.11
At present. case reports of bacteremia and endocarditis in humans resulting from infection with B. vinsonii10 and B. elizabethae are limited.13 Antibodies to B. elizabethae have been found in urban homeless and drug users,14 but the clinical significance remains poorly understood.
Disease in Animals
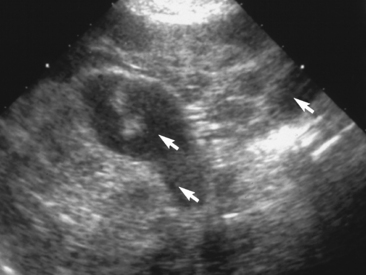
B. henselae causes subclinical infection in cats, including chronic bacteremia. Between 5% and 60% of cats may be seropositive depending on the geographical area. There is a case report of B. henselae infection in a Golden Retriever causing pelosis hepatitis (Figure 9-12 and Color Plate 9-4).15 Table 9-7 provides comparative clinical manifestations in humans and other animals.
Diagnosis
Diagnosis in humans is based on the clinical picture of local lymphadenopathy, especially in the setting of a history of cat contact. Bartonella species are difficult to grow in culture; therefore other diagnostic techniques such as PCR and serology are often required (e.g., immunofluorescent antibody [IFA] titer ≥1:64 to B. henselae).19 Cross-reactions can occur among Bartonella species and Chlamydia andCoxiella. PCR of tissue and fluid obtained from lymph node biopsy can often identify the organism. Disease in animals can be diagnosed by blood culture or serology (IFA or ELISA) and PCR.
Treatment
In humans, some cases of CSD in an immunocompetent host may not require antibiotic treatment.12 However, any immunocompromised patient, as well as any patient with extralymphatic involvement, should be treated with antibiotics.20 Table 9-8 outlines treatment guidelines for symptomatic disease in humans. There are no treatment protocols for animals.
Table 9-8 B. Henselae Treatment in Humans and Other Animals21
| Species | Primary Treatment | Alternative |
|---|---|---|
| Humans | ||
| Cat-scratch disease (immunocompetent) | Adults: azithromycin 500 mg × 1, then 250 qd × 4 days Children (≤45.5 kg): liquid azithromycin 10 mg/kg ×1, then 5 mg/kg/day × 4 days12 | Consider no treatment since often self-limited |
| Bacillary angiomatosis, peliosis hepatitis, immunocompromised patients | Clarithromycin 500 mg bid or clarithromycin ER 1 gm PO q24h or azithromycin 250 mg q24h or ciprofloxacin 500-750 mg PO bid × 8 wk12 | Erythromycin 500 mg PO qid or doxycycline 100 mg PO bid × 8 weeks, or if severe, combination of doxycycline 100 mg PO/IV bid and rifampin 300 mg PO bid |
| Cats, Dogs (With Clinical Signs) | Azithromycin 5-10 mg/kg once a day × 7 days and every other day × additional 5 weeks22 | |
1. Carr R.M., Mohrman L., Arelli V., et al. Update on cause and management of catscratch disease. Infect Med.. 2008;25:242.
2. Koehler J.E., Duncan L.M. Case records of the Massachusetts General Hospital. Case 30-2005. A 56-year-old man with fever and axillary lymphadenopathy. N Engl J Med.. 2005;353(13):1387.
3. Mandell G.E., Bennett J.E., Dolin R. Principles and practice of infectious diseases, 6th ed. Philadelphia: Churchill Livingstone Elsevier, 2000.
4. Juncker-Voss M., Prosl H., Lussy H., et al. Screening for antibodies against zoonotic agents among employees of the Zoological Garden of Vienna, Schönbrunn. Austriac.. 2004;117(9–10):404.
5. Breitschwerdt E.B., Kordick D.L. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev.. 2000;13(3):428.
6. Acha P.N., Szyfres B. Zoonoses and communicable diseases common to man and animals: vol. 2: chlamydioses, rickettsioses, and viroses, 3rd ed. Washington DC: Pan American Health Organization, 2003.
7. Fabbi M., Vicari N., Tranquillo M., et al. Prevalence of Bartonella henselae in stray and domestic cats in different Italian areas: evaluation of the potential risk of transmission of Bartonella to human beings. Parassitologia.. 2004;46(1–2):127.
8. Maurin M., Birtles R., Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis.. 1997;16(7):487.
9. Breitschwerdt E.B., Maggi R.G., Duncan A.W., et al. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis.. 2007;13(6):938.
10. Welch D.F., Carroll K.C., Hofmeister E.K., et al. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol.. 1999;37(8):2598.
11. Comer J.A., Paddock C.D., Childs J.E. Urban zoonoses caused by Bartonella, Coxiella, Ehrlichia, and Rickettsia species. Vector Borne Zoonotic Dis.. 2001;1(2):91.
12. Gilbert D.N., Moellering R.C., Ellopoulos G.M., et al. Sanford guide to antimicrobial therapy 2009, 39th ed. Sperryville, VA: Antimicrobial Therapy, 2009.
13. Daly J.S., Worthington M.G., Brenner D.J., et al. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol.. 1993;31:872.
14. Comer J.A., Diaz T., Vlahov D., et al. Evidence of rodent-associated Bartonella and Rickettsia infections among intravenous drug users from Central and East Harlem, New York. Am J Trop Med Hyg.. 2001;65(6):855.
15. Kitchell B.E., Fan T.M., Kordick D., et al. Peliosis hepatis in a dog infected with Bartonella henselae. J Am Vet Med Assoc.. 2000;216(4):519-523. 517
16. Dvorak G., Rovid-Spickler A., Roth J.A., et al. Handbook for zoonotic diseases of companion animals. Ames, IA: The Center for Food Security and Public Health, Iowa State University, 2008.
17. Roux V., Eykyn S.J., Wyllie S., et al. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J Clin Microbiol.. 2000;38(4):1698.
18. Fenollar F., Sire S., Raoult D. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J Clin Microbiol.. 2005;43(2):945.
19. Heymann D.L. Control of communicable diseases manual, 19th ed. Washington DC: American Public Health Association, 2008.
20. Boulouis H.J., Chao-chin C., Henn J.B., et al. Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet Res.. 2005;36:383.
21. Rolain J.M., Brouqui P., Koehler J.E., et al. Recommendations for treatment of human infections caused by Bartonella species. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=415619. Accessed September 11, 2008
22. Breitchwerdt E. Personal communication, August 21, 2008.
Brucellosis
Other names in humans: undulant fever, Mediterranean fever, Malta fever
Other names in animals: Bang’s disease (cattle), epizootic abortion, contagious abortion
Brucellosis is an important bacterial disease of ruminants worldwide and an occupational disease for humans working closely with infected animals. Many human cases are related to foodborne exposures to unpasteurized dairy products. Brucellosis prevention demands a “One Health” approach between animal and human health disciplines because the human health risk can be reduced only by controlling the disease in animals.1 Brucellosis can be passed between domestic cattle and wildlife such as bison, where it can be difficult to control.
Key Points for Clinicians and Public Health Professionals
Public Health Professionals
Human Health Clinicians
Veterinary Clinicians
Agent
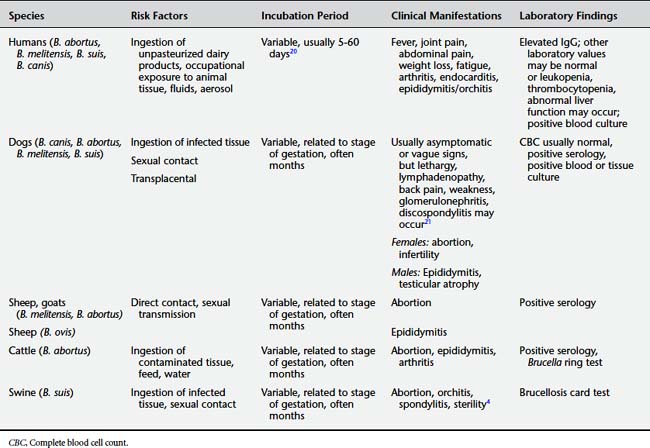
Brucellosis is caused by Brucella species, which are gram-negative coccobacilli. A number of species in the genus have affinities for particular animal hosts. These species have been further subdivided into biologically distinct strains (biovars).6 At least four species of Brucella are found in animals and man; these include Brucella abortus (cattle), B. melitensis (goats), B. suis (swine),* and B. canis (dogs). There have been several reported isolations of bacteria from marine mammals, including seals, whales, and dolphins that are currently classified in the genus Brucella. However, the human zoonotic potential of these new agents remains to be established.7
Geographical Occurrence
Brucellosis is found worldwide, but its prevalence depends largely on the state of control in domestic animals. In the United States, bovine brucellosis control programs have reduced the frequency of infection in both cattle and humans. Between 1986 and 2007, fewer than 150 human cases were reported annually in the United States.8,9 As a result, many human health clinicians in the United States have never seen a case. In the United States, the highest incidence rates are in states bordering Mexico, including California and Texas. The most common Brucella species for human infections in the United States are B. abortus and B. melitensis.10 The occurrence of human brucellosis is higher in other countries where livestock infection is not as well controlled, including Mexico, other Latin American countries, the Mediterranean basin, Eastern Europe, Asia, Africa, and the Middle East. The United Nations’ Food and Agriculture Organization has set a goal for worldwide eradication of brucellosis.
Groups at Risk
Worldwide, brucellosis is considered largely an occupational disease of workers exposed to cattle and other large animals through animal husbandry, dairy, and slaughter operations. Such workers include herdsmen, slaughterhouse workers, and veterinarians. Handling of infected aborted fetuses and newborn animals is considered a particularly high-risk activity. Another risk is sharing living spaces with potentially infected animals; high rates of brucellosis infection have been reported among goat-herding families that bring goats into family bedrooms during the winter.11
A rare occurrence among veterinarians is the self-inoculation (needlestick, splash or spray to mucous membranes, or broken skin) of vaccine strains of Brucella (RB51 or B. abortus strain 19, or B. melitensis Rev-1) during animal vaccination; this represents one of the rare reported occupational risks of animal vaccination.12 Outbreaks have occurred in laboratory workers who handled Brucella cultures outside biological safety cabinets,9 and dog handlers are considered to be at risk from B. canis. However, analysis of recent cases in the United States indicates that brucellosis is increasingly a foodborne disease associated with ingestion of unpasteurized dairy products from infected animals.10
Hosts, Reservoir Species, Vectors
The species and biovars of Brucella are adapted to particular animals that serve as definitive hosts. However, as shown in Tables 9-9 and 9-10, cross-species infections can occur, with humans and dogs particularly susceptible to infection by a number of different Brucella species.
Table 9-9 Hosts for Brucella Species
| Species | Animal Hosts |
|---|---|
| Brucella abortus | Cattle, elk,13 bison, water buffalo,14 goats,4 horses,15 dogs,4 coyotes16 |
| Brucella melitensis | Goats, sheep, cows, dogs4 |
| Brucella suis | Pigs, feral swine and wild boar, horses, cattle,4 dogs4 |
| Brucella ovis | Sheep |
| Brucella canis | Dogs |
Mode of Transmission and Life Cycle
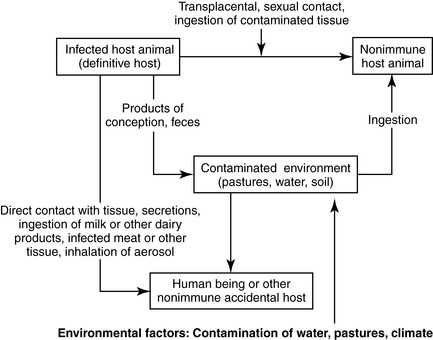
Brucellosis is transmitted by direct contact with infected tissues or secretions and enters the body by breaks in the skin or contact with mucous membranes (Figure 9-13). It can also be acquired by ingestion of contaminated foods. Aerosol transmission can easily occur. It is thought that a small number of inhaled organisms can lead to human infection, as seen in outbreaks of infection among laboratory workers. Brucella consequently requires biosafety level 3 containment in laboratories.
Transmission in cattle occurs through ingestion of contaminated pasture forage or water, as well as through licking and other direct contact of infected calves, fetuses, and afterbirths. Transmission in dogs is due to ingestion of or other contact with contaminated tissue, including sexual contact. The risk of transmission of brucellosis from dogs to humans is considered low and related to frequent close contact with blood, birth tissues, or other infected secretions.17 In humans, person-to-person infection has been reported through breastfeeding, childbirth, bone marrow transplants, sexual contact, and transfusions, but these modes of transmission are considered exceptional.6
Environmental Risk Factors
Brucella may survive for months in the environment in water and other media that are not exposed to direct sunlight or heat. Pastures can become contaminated by feces, products of conception, and vaginal discharges of infected animals, leading to spread of infection in herds of grazing animals. Brucella organisms have been recovered from cow manure that has remained in a cool environment for longer than 2 months.4
Disease in Humans
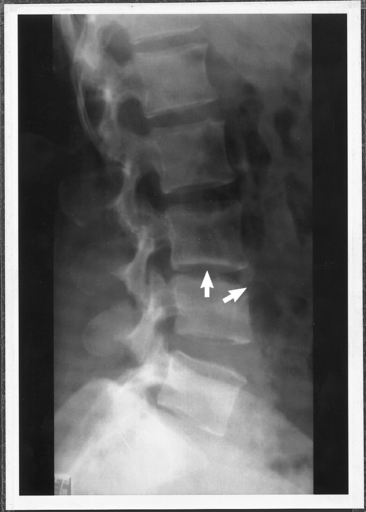
Five to 60 days after infection, Brucella can cause a febrile illness that may be abrupt or gradual. Symptoms are often nonspecific and include fever, headache, night sweats, fatigue, arthralgia, myalgia, joint pain, anorexia, abdominal pain, diarrhea, vomiting, and weight loss. Depression may be a prominent feature. The fever may be “undulant” in patients who remain untreated. Clinical findings may be unremarkable, but in 20% to 30% of patients hepatosplenomegaly and/or lymphadenopathy may develop. Osteoarticular involvement of the spine and large weightbearing joints (Figure 9-14) is common.18 Rarely, more severe infection can lead to epididymitis, orchitis, uveitis, endocarditis, and meningitis. A review of human cases found B. melitensis was more likely than B. abortus to cause abdominal pain and tenderness, hepatomegaly, splenomegaly, thrombocytopenia, pancytopenia, and hepatic dysfunction.10 Laboratory findings are usually mild or absent but may include elevation of liver function test results, thrombocytopenia, and other hematologic abnormalities.
Disease in Animals
In pregnant cows, B. abortus causes placental infection leading to abortion in the second half of gestation. It also causes reduced milk yield, testicular abscesses and epididymitis in bulls, and (rarely) joint involvement with long-standing infection.4 Infection in goats, sheep, and pigs is similar to that in cattle.
In sheep, B. ovis causes epididymitis in rams but abortions and stillbirths in ewes (Color Plate 9-5).4
Horses develop a rare bursitis condition known as poll evil or fistulous withers that may be caused by B. abortus or occasionally B. suis (Figure 9-15).4
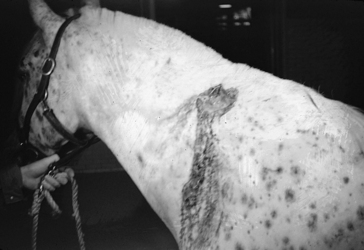
Figure 9-15 Fistulous withers in a horse.
(From Auer JA: Equine surgery, ed 3, Philadelphia, 2006, Saunders Elsevier.)
In dogs, the most common recognized sign and symptom are abortion and infertility. Females may have a prolonged vaginal discharge following abortion. Lymphadenitis may be seen. In males, orchitis, epididymitis, and prostatitis may occur. Spondylitis resulting in back pain and weakness, and uveitis are reported complications (Color Plate 9-6).4
Diagnosis
The differential diagnosis of brucellosis in humans is extensive and includes other causes of fever, including influenza, mononucleosis, human immunodeficiency virus (HIV), and malaria. A history of contact with animals, laboratory exposure, or consumption of unpasteurized dairy products should make clinicians suspect brucellosis. Diagnosis in humans is based on culturing the organism from blood, bone marrow, or other tissue, and/or serology. Cultures may be slow to grow and require caution in handling. Elevated immunoglobulin G (IgG) antibodies titers by ELISA or other tests including serum agglutination (SAT) are often key to the diagnosis, as active infection titers often exceed 1:160. Infection with B. canis may produce antibodies that do not react with standard Brucella test antigens; therefore specific B. canis serology must be requested if this infection is suspected. PCR techniques have shown promising results but are still in development.21
In dogs, cultures of blood and tissue are also used. There are several serological tests. The RSAT test has a high sensitivity but low specificity. The mercaptoethanol test also has low specificity; positive results must be confirmed by other tests such as the agar-gel immunodiffusion (AGID) test.4
Possible herd infection in cattle is diagnosed using the Brucella milk ring test, which is sensitive but not specific (Color Plate 9-7).4 Blood samples are collected from slaughtered animals. Further tests are used to confirm positive results.
Postexposure Prophylaxis
Following laboratory or vaccine exposure to Brucella species, 100 mg of doxycycline twice daily and rifampin 600 mg/day should be taken for 21 days. Trimethoprin sulfamethoxazole is an alternative for those with a contraindication to doxycycline. Doxycycline alone should be given if the exposure was to Brucella abortus strain RB51, which is resistant to rifampin.9 Baseline serum drawn for Brucella serology with repeat serology at 2, 4, 6, and 24 weeks can be used to monitor for evidence of infection. Such monitoring is not recommended for exposures to vaccine RB51, which does not elicit an antibody response on available assays. Exposed pregnant women should consult their obstetric care provider regarding PEP. Exposed persons should be monitored on a regular basis for the development of fever and other clinical signs of infection.9
Treatment
Antibiotic therapy for brucellosis infection in humans and other animals is outlined in Table 9-11 below. In humans, relapse and prolonged convalescence may occur after antibiotic treatment. Patients with focal complications including spinal or neurological involvement may require a more prolonged course of treatment.
Table 9-11 Brucellosis Treatment in Humans and Other Animals
| Species | Primary Treatment | Alternative |
|---|---|---|
| Humans (adult or child >8 yr23) | Doxycycline 100 mg bid × 6 wk PLUS gentamicin × 7 days or doxycycline 100 mg PO bid × 6 wk PLUS streptomycin 1 gm IM q24h × 2-3 wk | Doxycycline 100 mg PO bid PLUS rifampin 600-900 mg PO q24h × 6 wk or trimethoprim-sulfamethoxazole 1 DS tab (160 mg TMP) PO qid × 6 wk PLUS gentamicin × 2 wk |
| Humans (child <8 yr) | Trimethoprim-sulfamethoxazole 5 mg/kg PO q12h × 6 wk PLUS gentamicin 2 mg/kg IV/IM q8h × 2 wk23 | |
| Postexposure prophylaxis | Doxycycline 100 mg PO bid PLUS rifampin 600 mg PO qd × 3 wk (doxycycline alone if exposed to strain B. abortus RB51)9 | Trimethoprim-sulfamethoxazole (160 mg/800 mg) × 3 wk |
| Dogs | Doxycycline 12-15 mg/kg PO q12h × 4 wk PLUS gentamicin 5 mg/kg SC q24h at 0 and 1 wk21 | |
1. Zinsstag J., Schelling E., Roth F., et al. Human benefits of animal interventions for zoonosis control. Emerg Infect Dis.. 2007;13(4):527.
2. Food and Agriculture Organization (FAO). Guidelines for coordinated human and animal brucellosis surveillance. http://www.fao.org/docrep/006/y4723e/y4723e00.htm. Accessed September 4, 2008
3. Dvorak G., Rovid-Spickler A., Roth J.A., et al. Handbook for zoonotic diseases of companion animals. Ames, IA: The Center for food security and public health, Iowa State University College of Veterinary Medicine, 2008.
4. Kahn C.M., Line S., editors. The Merck veterinary manual, 9th ed., Whitehouse Station, NJ: Merck, 2005.
5. Kreeger T.J., DeLiberto T.J., Olsen S.C., et al. Safety of Brucella abortus strain RB51 vaccine in non-target ungulates and coyotes. J Wildl Dis.. 2002;38(3):552.
6. Acha P.N., Szyfres B. Zoonoses and communicable diseases common to man and animals: vol. 1: bacterioses and mycoses, 3rd ed. Washington, DC: Pan American Health Organization, 2001.
7. Godfroid J., Cloeckaert A., Liautard J.P., et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res.. 2005;36(3):313.
8. Chang M.H., Glynn M.K., Groseclose S.L. Endemic, notifiable bioterrorism-related diseases, United States, 1992–1999. Emerg Infect Dis.. 2003;9:556.
9. Centers for Disease Control and Prevention. Laboratory-acquired brucellosis—Indiana and Minnesota, 2006. MMWR Morb Mortal Wkly Rep.. 2008;57(2):39.
10. Stephanie B., Troy S.B., Rickman L.S., et al. Brucellosis in San Diego epidemiology and species-related differences in acute clinical presentations. Medicine.. 2005;84:174.
11. Acha P.N., Szyfres B. Zoonoses and communicable diseases common to man and animals: vol. 1: bacterioses and mycoses, 3rd ed. Washington DC: Pan American Health Organization, 2001.
12. Berkelman R.L. Human illness associated with use of veterinary vaccines. Clin Infect Dis.. 2003;37(3):407.
13. Rhyan J.C., Aune K., Ewalt D.R., et al. Survey of free-ranging elk from Wyoming and Montana for selected pathogens. J Wildl Dis.. 1997;33:290.
14. Nishi J.S., Stephen C., Elkin B.T. Implications of agricultural and wildlife policy on management and eradication of bovine tuberculosis and brucellosis in free-ranging wood bison of northern Canada. Ann N Y Acad Sci.. 2002;969:236.
15. Acosta-Gonzalez R.I., Gonzalez-Reyes I., Flores-Gutierrez G.H. Prevalence of Brucella abortus antibodies in equines of a tropical region of Mexico. Can J Vet Res.. 2006;70:302.
16. Davis D.S., Boeer W.J., Mims J.P., et al. Brucella abortus in coyotes. I. A serologic and bacteriologic survey in eastern Texas. J Wildl Dis.. 1979;15(3):367.
17. Centers for Disease Control and Prevention. Brucellosis. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/Brucellosis_g.htm#mydog. Accessed July 27, 2008
18. Mandell G.E., Bennett J.E., Dolin R. Principles and practice of infectious diseases, 6th ed. Philadelphia: Churchill Livingstone, 2000.
19. Colville J., Berryhill D. Handbook of zoonoses: identification and prevention. St Louis: Mosby Elsevier, 2007.
20. Heymann D.L. Control of communicable diseases manual, 19th ed. Washington DC: American Public Health Association, 2008.
21. Barr S.C., Bowman D.D. 5-Minute veterinary consult clinical companion canine and feline infectious disease and parasitology. Baltimore: Wiley-Blackwell, 2006.
22. Vrioni G., Pappas G., Priavali E., et al. An eternal microbe: Brucella DNA load persists for years after clinical cure. Clin Inf Dis.. 2008;46(12):e131.
23. Gilbert D.N., Moellering R.C.Jr, Eliopoulos G.M., et al. The Sanford guide to antimicrobial therapy, 2009, 39th ed. Sperryville, VA: Antimicrobial Therapy, 2009.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree