CHAPTER 19 WILDLIFE
DECIDING WHETHER OR NOT TO TREAT WILDLIFE
There are many financial, ethical, and emotional issues for veterinarians to consider when deciding whether to accept wildlife cases to their practice. Wildlife is not owned and therefore do not come with paying caretakers. In many cases, the hospital will be expected to absorb the cost of treatment, although avenues for monetary compensation, including grants and public donations, do exist. Accepting wildlife cases is often perceived by (prospective) clients as a positive reinforcement of a veterinarian’s compassion toward animals and can serve, directly or indirectly, as a method of increasing a veterinarian’s domestic and exotic pet caseload. One ethical consideration to make with these cases is deciding when intervention may interfere with a natural process occurring in a population. In some cases, euthanasia will certainly end suffering, whereas in others treatment may interfere with both natural selection and the disease status in a population. However, because the lives of humans have become so enmeshed with populations of wild animals, it is unavoidable that some, if not most, of the injuries or disease processes occurring in wildlife populations are the direct result of human contact. In these cases, there may be a strong moral (ethical) obligation to repair damage caused by the human species. An early and rational assessment of the likely return to function for a patient is imperative to conserve the available resources for treatment and prevent undue suffering in the patient. Animals who are considered poor candidates for release or placement should be considered for euthanasia as soon as an assessment is made. Release candidates should be able to function appropriately within their natural habitat (including reproduction) and with conspecifics; otherwise, a disservice is rendered to both the animal and its population.
KNOWING THE REGULATIONS
The federal government is primarily invested in the protection of migratory birds and all other species that are listed federally as species of special concern, threatened, or endangered. State agencies also require rehabilitators to maintain permits to cover these same species, as well as mammals and other species (reptiles, amphibians, and fish) considered threatened or endangered at the state level. Veterinarians interested in obtaining federal permits can find information regarding the permits on the United States Fish and Wildlife Service website (www.fws.gov). Veterinarians interested in obtaining state rehabilitation licensure should contact their state wildlife and fisheries service.
PREPARING FOR WILDLIFE IN VETERINARY HOSPITALS
Staff Preparedness
When veterinarians accept wildlife cases into their hospitals, it is important that they have buy-in from their staff. Wildlife cases can require a significant amount of time and effort, especially orphaned animals. Therefore, it is often the veterinary staff that will have the most contact with the wildlife cases. Veterinarians that accept these types of cases on a moderate to large scale would certainly benefit from providing at least some of their staff an opportunity to obtain continuing education in wildlife rehabilitation. Many of these opportunities can be obtained from local or state meetings, although the large national meetings (e.g., the annual conferences of the National Wildlife Rehabilitators Association and the International Wildlife Rehabilitators Association) provide the most diverse training opportunities. Membership in these organizations is also highly recommended, as they publish regular bulletins or journals and provide a real resource of potential personal contacts.
It is important that veterinarians train their staff to avoid becoming emotionally attached to their wildlife patients. In our opinion, this is the single most controversial issue that can arise in a hospital. If staff members (and veterinarians) become emotionally attached to their cases, they are less likely to maintain an objective outlook on their cases. It is important to always approach these cases with a triage mentality (Figure 19-1). The time spent treating these patients should also be limited to necessary contact only. Animals that become habituated to humans in captivity may be at greater risk of reinjury or death after release if they lose their fear of humans. For orphaned animals, imprinting is a major concern. Imprinting occurs when an animal recognizes a human as the “parent” animal. Imprinting can potentially lead to dangerous encounters for humans and wildlife, especially with mesopredators, carnivores, and raptors. For this reason, it is important that only trained staff manage orphaned animals.
Equipment
Most of the medical and surgical equipment required for wildlife patients is already available in veterinary hospitals that treat domestic species. However, if a practice is primarily based on domestic species, there may be some specialty equipment that needs to be obtained. A list of different equipment we consider important can be found in Box 19-1.
HOUSING
Avian
GAMEBIRDS
Principles of short-term and long-term housing are the same for these birds as was described for pigeons and doves, although the size of the cage may need to be increased for larger specimens. No perching materials need to be provided for short-term housing but should be provided if the bird is kept for a longer period of time to allow roosting at night.1 Substrate should be easily disinfected, to prevent foot disease (e.g., Astroturf is a good substrate).
WADERS
Short-term housing for waders should be tall enough to enable the animal to stand naturally. The cage should also be kept dark and quiet with moist, nonslip flooring (e.g., moist sand or newspaper).2 Water should be provided in a shallow container. For long-term housing, a shallow pond or pool and suitable natural cover should be provided. As with waterfowl, this may be difficult for general practitioners to provide.
RAPTORS
All of the general principles for housing apply to raptors. In addition, it is extremely important to provide an environment that will prevent any self-induced trauma. Raptors are prone to damaging their feathers, wings, talons, and cere in captivity. Injuries to any of these body parts can delay the rehabilitation process and should be avoided at all costs. The best cage materials to use for housing raptors are those that do not bend or damage the feathers. Because most veterinary hospitals maintain chain-link fence kennels or stainless steel cages, housing for raptors may be limited. Covering the cage with a soft mesh can help diminish the amount of feather damage done to hospitalized raptors. Tailguards made out of old radiograph film can be used to reduce tail feather damage. Perches can also be used to help limit the amount of damage done to the tail feathers. The perch should be positioned so that the tail feathers do not touch the base of the cage and the flight feathers have minimal contact with the sides of the cage. For extended hospitalization, raptors should be housed in an appropriately sized aviary. Whereas groups of some species can be housed together, mixing raptors is not recommended. As with short-term housing, care must be taken that the animal cannot injure itself. An easily disinfected substrate, such as Astroturf or gravel, can be used for the aviary. Multiple perches should be provided. If the bird is not a solid flier, the perches should be maintained close to the ground. Covering the perches with a rough surface (e.g., Astroturf) will minimize the likelihood of a bird developing pododermatitis. Multiple diameter and textured perches should be placed in the cage; however, the diameter should not be so small that the talons puncture the plantar surface of the foot.3 The perches should be cleaned regularly to minimize the likelihood of fecal contamination. As with other species, hay or straw should not be used because of the potential for exposure to Aspergillus spp. spores. Certain species, such as owls, will benefit from shelter.
Mammals
DEER
For short-term hospitalization, fawns can be kept in an isolation area or any area that is away from the noise of humans and other animals. Large deer can be kept for short periods of time in a stable or outhouse with a deep layer of straw. As for the size of the enclosure, a maximum of 1.5 × 1.8 m and 2 × 3 m for fawns and adult deer, respectively, is recommended.4 The enclosure should not have windows, and ventilation should be provided through slats at the top of the walls or ceiling. A door that is divided into a stable-door arrangement, with the top portion only 0.5 m high, is preferred. It is best if the door can be swung inward, so that it can be used as a restraint device for the deer. The top portion of the door should be opened for access only from above. Deer will leap toward light when the door is opened, making other arrangements dangerous. Deer who will be kept in captivity for longer periods of time should be transferred to an experienced rehabilitator who has appropriate pens.
QUARANTINE
Quarantine is an important consideration when practicing wildlife medicine in a domestic species veterinary hospital. Unfortunately, most veterinary hospitals are not built with quarantine in mind. Because wildlife can harbor various bacterial, fungal, viral, and parasitic diseases that can be transmitted to domestic pets, it is important to minimize and restrict both direct and indirect contact between these animals. Wildlife should be housed in a separate room from domestic species. A room with its own ventilation system is preferred. The traffic through the wildlife ward should be one-way. A foot bath with a disinfectant (e.g., sodium hypochlorite) should be placed outside the doorway and used after exiting the room to minimize the likelihood of tracking infectious diseases throughout the hospital. The disinfectant should be changed daily, or as often as needed, to minimize the amount of organic debris in the solution. Organic debris renders many disinfectants useless. Laboratory coats, or preferably jumpsuits that totally cover clothing, should be placed in the wildlife ward and worn when working with the animals. These clothing materials should be taken out of the room only for washing. Any materials being removed from the ward should be placed in a garbage bag and carried through the hospital in these bags to minimize the likelihood of disseminating disease through the hospital. A hand-washing station should be placed within the room. Signs should be posted on the wildlife ward door to alert staff and clients about the presence of wildlife and the need to maintain a strict quarantine protocol.
ZOONOSES
Zoonotic diseases should always be a concern for those individuals working with wildlife, as many of these animals can harbor a variety of bacterial, viral, fungal and parasitic zoonotic agents (Box 19-2). Because of this, children and individuals with compromised immune systems should not be allowed to work with wildlife. Staff should not be allowed to eat, drink, or smoke near any wildlife patients, and food and drink items should not be allowed near any of the places where diagnostic samples are held. We strongly recommend wearing examination gloves when handling wildlife. Wearing gloves will help minimize the likelihood of introducing pathogens into cuts or abrasions on practitioners’ and staff’s hands. For those cases where an aerosolized pathogen is suspected, a protective mask and eyeglasses should be worn. Individuals who develop an illness after working with wildlife should be examined by a health care specialist immediately. Zoonoses and Communicable Diseases Common to Man and Animals (ed 3), coedited by P. N. Acha and B. Szyfres and published by the Pan American Health Organization (Washington DC), is an excellent reference for obtaining additional information on zoonotic diseases associated with wildlife.
EMERGENCY CARE
Cardiopulmonary Resuscitation
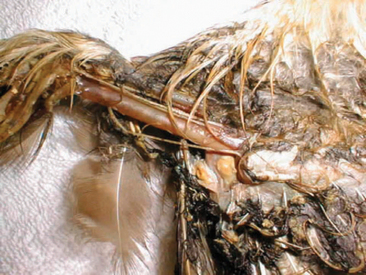
The first steps to performing cardiopulmonary resuscitation are to intubate the patient and initiate cardiac compressions. In most birds and reptiles, the glottis is located at the base of the tongue and can be easily visualized and intubated (Figure 19-2). The choice of endotracheal tube should be commensurate with the animal’s tracheal diameter. For small species, intravenous catheters (14- to 16-gauge) may be modified into an endotracheal tube. Because birds, crocodilians, and chelonians have complete tracheal rings, the cuff on their endotracheal tube should not be insufflated. In mammals, the intubation procedure can be more difficult, especially in rabbits; however, a laryngoscope or endoscope can be used to assist with intubation. In those cases where intubation is difficult, if not impossible, the animal should be masked and a high flow of oxygen provided. The success rate for animals that cannot be intubated will be less than 1%. Respirations can be given every 5 to 30 cardiac compressions.
Thermoregulation
The body temperature of a patient can provide a significant amount of information regarding its physiologic status. Although birds are endotherms, there are times when the provision of supplemental heat is important. Birds that fluff their feathers do so to maintain body heat. Birds displaying this behavior must convert energy to maintain their core body temperature. To confirm this, the animal’s body temperature should be taken. Birds naturally maintain a higher body temperature than mammals, with most birds falling between 104° F and 108° F. Because birds with an illness must conserve energy, it is important that veterinarians provide supplemental heat to minimize the energy expenditure associated withheat conservation. Temperature-controlled incubators provide an excellent method of providing a consistent environmental temperature (Figure 19-3). Heating pads, hot water bottles, and incandescent lights can also be used to provide supplemental heat, but all should be monitored closely. Adult birds that are hypothermic can be maintained in an environmental temperature of 82° F to 88° F, whereas juvenile birds may need to be kept warmer depending on the extent of their feather coverage (e.g., nestling vs. fledgling). Mammals, like birds, are also endothermic, and although they can control their own body temperature, they may require supplemental heat on occasion. Mammals that are hypothermic may shiver, have cold extremities, and have a body temperature below the acceptable reference range. Most mammalian body temperatures are between 100° F and 104° F. Opossums are an exception, generally having a body temperature between 92° F and 96° F. Reptiles are ectotherms and depend on their environmental temperature to regulate their core body temperature. For North American reptiles, a temperature range of 80° F to 90° F is appropriate.
Fluid Therapy
Fluid therapy should be provided next. The volume of replacement fluids required for a patient can be calculated by determining the maintenance rate of fluids for a day and degree of dehydration. A list of maintenance fluid rates for different types of wildlife is found in Table 19-1. The techniques used to assess dehydration can be found in Table 19-2. Fluids should always be warmed to the patient’s physiologic temperature before being administered. Identifying which fluid is most appropriate will be based on the type of dehydration. Most wildlife cases present with isotonic dehydration, so a balanced fluid (e.g., 0.9% saline, lactated Ringer’s solution) can be used. Calculating the patient’s osmolality is the best method for estimating the type of dehydration. Laboratories using osmometers can calculate this number; however, if submitting a sample is not possible, then an estimate can be made using the following mathematical formula: OsM = 2(sodium + potassium) + urea + glucose. For reptiles and birds, uric acid can be used as a substitute for urea; however, it may underestimate the true osmolality. A fluid deficit in a mammalian patient can be corrected within 24 to 36 hours, whereas in birds and reptiles, it may take 48 and 96 hours, respectively.
TABLE 19-1 Maintenance Fluid Rates for Wildlife Patients
| Group | Suggested maintenance fluids |
|---|---|
| Avian | 75-100 ml/kg/day |
| Mammals | 80-100 ml/kg/day |
| Reptiles | 10-30 ml/kg/day |
TABLE 19-2 Estimating Dehydration in Wildlife
| Percentage dehydrated | Clinical signs |
|---|---|
| 5% | Some loss of skin turgor |
| Skin slow to return after tenting (2-3 sec) | |
| Some tackiness in mucous membranes | |
| Capillary refill time (<2 sec) | |
| 7%-8% | Moderate loss of skin turgor |
| Skin slow to return after tenting (3-5 sec) | |
| Ropy mucus in oral cavity, dry mucous membranes | |
| Capillary refill time (<3 sec) | |
| >10% | Significant loss of skin turgor |
| Skin very slow to return after tenting (>5 sec) | |
| Significant ropy mucus in oral cavity | |
| Capillary refill time (>3 sec) | |
| Sunken eyes (loss of retroorbital fat pads) |
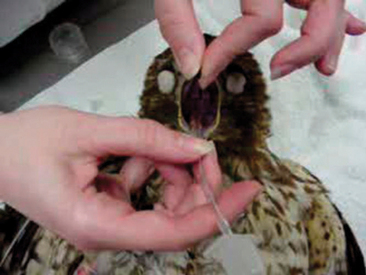
Fluids can be delivered to wildlife using the same basic techniques described for domestic pets. Animals that are only mildly dehydrated and have a functional gastrointestinal tract can have fluids delivered per os. Subcutaneous fluids can be used for those patients that are mildly dehydrated but have some gastrointestinal disease (e.g., diarrhea, vomiting). For moderately dehydrated reptile and mammalian patients, fluids can be given intracoelomically or intraperitoneally, respectively. Because birds have air sacs, they should never be given fluids intracoelomically. For moderate to severe dehydration, fluids should be given intravenously (Figure 19-4) or intraosseously.
Fracture and Wound Management
Fractures should be stabilized to minimize the risk of further injury to the patient and to control pain. Depending on the site and nature of the fracture, various emergency bandaging methods can be employed. We generally immobilize wing fractures with a figure-of-eight splint (Figure 19-5) or a body wrap. The figure-of-eight splint is appropriate for fractures distal to the elbow, and a body wrap is recommended when the fracture includes the humerus. Vet-wrap (3M Corp., St. Paul, MN), or another comparable bandage material, can be used. For small birds (e.g., hummingbirds), placing the wings in a normal position and taping the primaries where they cross can provide an adequate splint. When immobilizing the wings of a bird, it is important to change the bandage and provide physical therapy every 3 to 4 days. This will prevent excessive contraction of the patagial tendon and atrophy of the muscles. We prefer to replace the bandage under anesthesia, as this minimizes the likelihood of the animal reinjuring itself and controls the pain associated with the procedure. In birds, fractures of the legs can be immobilized with modified syringe-case splints, tape splints, a ball bandage, or a Robert Jones–type splint. Syringe case splints are best suited for fractures distal to the stifle. Tape splints can be used to stabilize toes or the distal leg of a passerine or other lightweight bird. We generally use white, porous tape for these splints. For mammals, limbs can be splinted using the standard techniques described for domestic mammals. Fractures of the reptile limb can be immobilized by taping the limb to the animal’s body. In lizards, a forelimb can be secured against the body wall using porous tape while the rear leg can be immobilized against the tail. In chelonians, the limb can be reduced into a normal position within the shell and taped into place. When splinting any limb, it is important to always immobilize the joint above and below the fracture site.
THERAPEUTICS
Once fluid therapy is initiated, chemotherapeutics can be given. It is important to wait on certain drugs until after fluid therapy has been instituted. For example, the administration of steroidal antiinflammatories could have a negative response if given to a dehydrated patient. A variety of chemotherapeutics are available to the veterinarian, including antiinflammatories (steroidal and nonsteroidal), analgesics, antibiotics, antifungals, antivirals, anesthetics, antiemetics, chelating agents, vitamins, and minerals. A list of common drugs used to treat wildlife can be found in Table 19-3.
TABLE 19-3 Drugs Commonly Used to Treat Wildlife
| Drug | Dose | Comments |
|---|---|---|
| Emergency drugs | ||
| Atropine | 0.5 mg/kg | IV, IO, IT |
| Diazepam | 0.5-1.0 mg/kg | IV, IO |
| Doxapram | 20 mg/kg | IV, IO, IT |
| Epinephrine | 0.5-1.0 mg/kg | 1 : 1,000; IV, IO, IT |
| Antibiotics | ||
| Amikacin | 3-5 mg/kg | Reptiles: IM, q72h |
| 15-20 mg/kg | Birds: IM, q24h | |
| 2.5-10 mg/kg | Mammals: IM, q12-24h | |
| Amoxicillin | 10-30 mg/kg | Reptiles: PO, IM, q12-24h |
| 100 mg/kg | Birds, PO, q12h | |
| 10-20 mg/kg | Mammals: PO, q12h; NOT recommended for rodents or lagomorphs | |
| Cephalexin | 20-40 mg/kg | Reptiles: PO, q12h |
| 100 mg/kg | Birds, PO, q12h | |
| 15-30 mg/kg | Mammals: PO, q12h; NOT recommended for rodents or lagomorphs | |
| Ceftazidime | 20 mg/kg | Reptiles: IM, q72h |
| Ciprofloxacin | 10 mg/kg | Reptiles: PO, q24h |
| 15 mg/kg | Birds: PO, q12h | |
| 15 mg/kg | Mammals: PO, q12h | |
| Doxycycline | 5-10 mg/kg | Reptiles: PO, q24h |
| 25-100 mg/kg | Birds: PO, q24h | |
| Enrofloxacin | 5-10 mg/kg | Reptiles: PO, q24h |
| 10-15 mg/kg | Birds: PO, q12h | |
| 5-10 mg/kg | Mammals: PO, q12h | |
| Tetracycline | 200 mg/kg | Birds: PO, q12-24h |
| 20 mg/kg | Mammals: PO, q12h; use with caution in lagomorphs and rodents | |
| Trimethoprim-sulfadiazine | 15-30 mg/kg | Reptiles: PO, q24h |
| 50-75 mg/kg | Birds: PO, q12-24h | |
| 5-25 mg/kg | Mammals: PO, q12-24h | |
| Antifungals | ||
| Itraconazole | 5-10 mg/kg | Reptiles: PO, q24-48h; monitor for neurologic disease |
| 2.5-10 mg/kg | Birds: PO, q24h; monitor for neurologic disease | |
| 2.5-5 mg/kg | Mammals: PO, q24h; monitor for neurologic disease | |
| Ketoconazole | 25-50 mg/kg | Reptiles: PO, q24h |
| 20-50 mg/kg | Birds: PO, q12-24h | |
| 20-50 mg/kg | Mammals: PO, q24h | |
| Nystatin | 100,000 IU/kg | Reptiles: PO, q24h |
| 100,000-300, 000 IU/kg | Birds: PO, q12h | |
| Antiparasitics | ||
| Fenbendazole | 25-100 mg/kg | Avian, reptiles: PO, once, repeat 10-14 days |
| 5-10 mg/kg | Mammals: PO, q24h × 3-5 days | |
| Ivermectin | 0.2-0.4 mg/kg | All wildlife: IM, PO; Do not use in chelonians |
| Metronidazole | 25-50 mg/kg | Reptiles: PO, q24-48h |
| 20-40 mg/kg | Birds: PO, q12-24h | |
| 15-25 mg/kg | Mammals: PO, q12-24h | |
| Praziquantel | 5-8 mg/kg | Reptiles: IM, repeat 10-14 days |
| 20-40 mg/kg | Birds: PO, q12-24h | |
| 5-10 mg/kg | Mammals: IM, repeat 10-14 days | |
IM, Intramuscular; IO, intraosseous; IT, intratracheal; IV, intravenous; PO, per os.
CAPTURE AND TRANSPORT
An appropriate-sized, darkened cardboard box (with ventilation holes cut in) or pet carrier that opens from the top can be used to transport wildlife. The cardboard box is an excellent transport carrier because it can be discarded after use; the pet carrier can be disinfected between uses. Homemade wooden boxes may also be used but are more difficult to disinfect. It is important to include a nonslip floor covering in the transport container to ensure proper footing in the transport box. Noncovered cardboard surfaces have been associated with the development of splay leg and bilateral partial paresis in some bird species.5 When transporting birds, it is imperative that the box be large enough that the bird can turn without damaging its feathers. When flight or tail feathers are damaged, it can increase the rehabilitation time for a patient.
Avian Species
SEABIRDS
The main danger posed by seabirds is from their beaks, which they may use to bite or stab at the face. Some seabirds also have sharp claws, which are capable of causing deep lacerations. As with waders, safety goggles should be worn and the bird never restrained close to the face. If the species in hand has internal nares, be careful not to hold the beak closed because it can affect normal respiration. These birds will also attempt to return to the water when they sense danger, so care must be taken to block this route of escape. A net is usually necessary for capture, but a towel or blanket may also be used. The beak can be kept closed by using tape or a rubber band. The head of these animals should be restrained and covered at all times, to prevent injury to the handler and reduce the stress on the animal. A towel can be wrapped around the body of these birds to restrain the wings; however, the towel should not be held so tight that it affects respiration (e.g., keel excursions). Because seabirds commonly regurgitate, it is important to remove any beak restraint before transporting the animal, to prevent aspiration.5 The transportation carrier must be large enough to prevent damage to the flight and tail feathers.
RAPTORS
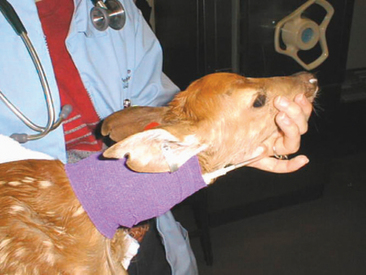
Although the beaks of raptors may look formidable, the biggest threat associated with these birds is their talons. Thick leather gloves, such as welding gloves, should be worn when handling medium to larger-sized specimens. Small raptors may be handled using outdoor work gloves (e.g., cotton or leather). When approached, most raptors roll backward and present their talons. When presented with a bird in this position, it is possible to offer them a towel or blanket to grasp before restraining the legs. The legs should always be grasped as close to the body wall as possible, as this will reduce the likelihood of causing a tibiotarsal fracture or injuries associated with self-taloning. The head of the raptor should be grasped using the same techniques to restrain other birds, with the index finger and thumb positioned under the mandible (Figure 19-6). For animals that are free-ranging, a bal chatri trap may be used. These traps can be built out of wire mesh (e.g., chicken wire) and fishing line. The wire mesh is first used to construct a wire cage to hold a live prey species (e.g., pigeon or rodent). A second piece of chicken wire is then placed over the prey cage, thus preventing injury to the prey species. The fishing line is then cut into small pieces (6-8 cm) and tied into small nooses. These nooses are secured over the entire surface of the trap. When the raptor attacks the trap, it becomes entangled in the trap and cannot lift off the ground with the trap.
Mammals
Unconscious mammals must be approached and handled with caution, as they may suddenly regain consciousness. Mammals should only be carried in an appropriate transport container and should never be captured or handled with unprotected hands. For capture, a towel or blanket may be placed over the animal to reduce its vision. Large and potentially dangerous mammals should always be sedated before being transported.
LAGOMORPHS
Although considered docile by many, lagomorphs may bite and scratch during capture and handling. Leather gloves should be worn to reduce the risk of injury. Rabbits and hares are more susceptible to the effects of stress than most other species. The short-term effects associated with stress may be manifested in sudden death due to heart failure or fatal oliguria.6 Over longer periods of time, stress may be manifested as reduced gut motility and the disrupted carbohydrate metabolism, leading to diarrhea, hepatic lipidosis, liver failure, and death. In these cases, the cecal microflora are also disrupted, leading to enterotoxemia and gut stasis. Lagomorphs are also prone to spinal injury during capture and handling.6 Blankets, towels, or nets may be used to capture animals that are free-ranging.
MESOPREDATORS: OPOSSUMS, RACCOONS, AND FOXES
Mesopredators have very sharp teeth and can deliver a painful bite, even through leather gloves. Animals that are severely ill or in shock may appear tame but can still inflict a serious injury. It is important to know that opossums can climb upward when held by their tail. Mesopredators may be caught in a net and then pinned using a soft-headed broom. Once pinned safely, they can be scruffed. If this method does not work, a quick-release rabies pole can be used. It is preferable to use one with a rubber end to prevent fracturing an animal’s teeth as is bites the pole.7 Inexperienced individuals should not attempt to capture these animals, as they are likely to injure both the animal and themselves. A live animal trap, plastic domestic pet travel carrier, or sturdy plastic trash can with a tight-fitting lid can be used to transport mesopredators.
DEER
Yearling and adult deer can pose a special danger. These animals are capable of gouging with their antlers or kicking with their feet. Only trained professionals should attempt to capture an injured subadult or adult deer. A deer that is severely injured or in shock may appear tame when approached; however, they are still capable of quick bursts of speed and energy and can injure an inexperienced handler. Before approaching an injured deer, it should be evaluated from a distance to determine its general status. The equipment generally used to capture deer include dark towels or drapes for covering the head, thick blankets, soft ropes, soft cargo or freight netting, and possibly a dart gun or pole-syringe.4 Anesthetics appropriate for sedating deer and a euthanasia solution should be kept on hand. If the deer is trapped in fencing or some other physical structure, it should be sedated remotely to prevent further injury to the deer. Expert assistance should also be sought for transport of injured deer. Individual deer should never be transported loose within a trailer without restraint. Large deer can best be transported wrapped up in cargo nets with the eyes blindfolded and the feet hobbled. Small deer can be wrapped in blankets. Trussed-up deer can be transported in a vehicle or trailer.
RESTRAINT AND HANDLING
Manual
Once the animal is transported to the clinic, the veterinary staff should be prepared to restrain and handle the animal for the physical examination and any diagnostic testing procedures. It is essential that the staff be trained properly to minimize the likelihood of injury to the human handler and patient. Restraint and handling is a stressful time for the patient, often leading to elevated cortisol or corticosterone production. Over time, this physiologic stress could have detrimental effects on the patient, including a reduction in metabolism, immune function, and normal behaviors.