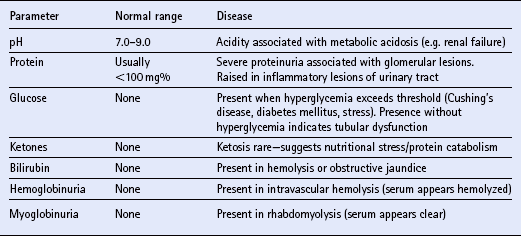
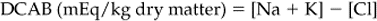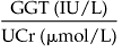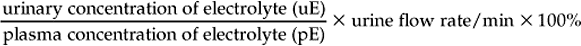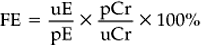Chapter 12 The upper urinary tract comprises the kidney and the ureters. The kidneys are responsible for the fine-tuning of the water and electrolyte balances, and the amount of water ingested or produced metabolically can have a significant effect on urine production. The ureters conduct the urine to the bladder in a pulsatile fashion with muscular contractions delivering accumulated urine from the renal pelvis to the bladder up to 6–10 times per minute. Bladder function is governed by sympathetic, parasympathetic and somatic nerves. Throughout filling, afferent (sensory) neurons induce increased sympathetic activity, which inhibits detrusor contraction. Passive outflow of urine from the urinary bladder is prevented by muscular tone in the neck of the bladder and the proximal urethra. Bladder distension triggers micturition by coordinated contraction of the bladder detrusor muscle, which generates intravascular (bladder) pressure, and simultaneous relaxation of the bladder and urethral “sphincters”. Higher centers in the motor cortex, midbrain and medulla can both inhibit and facilitate contraction by over-riding the automatic local functions. In this way urination can be consciously delayed. IV pyelography/excretory urograms can be performed in adult horses and are used mainly to identify ectopic ureters. Suitable contrast medium (e.g. iohexol) is administered by slow IV injection (at a rate of 1 mL/kg body weight) via a jugular catheter. The first radiographs are taken at 3 min and then sequential views are taken at 2 min intervals thereafter for 6–10 min. By this stage the contrast should be clearly visible in the renal pelvis and passing down the ureters. An ectopic ureter will be seen to discharge into an abnormal site (e.g. the anterior vagina) whereas in the normal ureter(s) the contrast will accumulate in the bladder and be clearly visible. The technique has limitations of size and facilities to take diagnostic lateral radiographs. Combinations of positive and negative contrast can be helpful also but these can be logistically more difficult. Azotemia is a laboratory-derived term used when there are increased blood concentrations of urea and creatinine (and other non-protein nitrogen) in blood. The term uremia (uremic syndrome) is used to describe the widespread effects of high concentrations of urea (azotemia) on body tissues. There is a poor correlation between the severity of uremia and the laboratory-derived concentrations of urea and creatinine. The extent of azotemia is usually highest with renal and post-renal azotemia but the best approach is probably achieved by comparing the blood and urinary creatinine and urea concentrations: 1. A urine to blood creatinine ratio >50:1 (reflecting concentrated urine) would be expected in pre-renal azotemia. 2. Urine to blood creatinine ratios <37:1 probably indicate renal disease. 3. A high urinary specific gravity (>1.035) is usually present in pre-renal azotemia. 4. Dilute urine in the face of dehydration is consistent with a diagnosis of renal disease. Hypoalbuminemia arises from protein loss in the urine. Tables 12.1 and 12.2 list the chemical and sedimentary characteristics of normal urine and the changes associated with disease. It is important to make the distinction between physiologic and pathologic changes in color. Normal horse urine varies from pale yellow to almost colorless. The yellow coloration is due to a normal pigment (urochrome) and its intensity can vary in proportion to the specific gravity. Water deprivation results in the excretion of concentrated urine with a higher specific gravity and more intense color. Physiologic dilution of urine can occur with psychogenic polydipsia (q.v.) and with excessive fluid therapy. Table 12.2 Sedimentary characteristics of normal equine urine and changes associated with disease The three most common alterations of urine color from the normal pale yellow color are listed in Box 12.2. 1. Hematuria (whole blood in urine) 2. Hemoglobinuria (hemoglobin in urine) 4. Certain drugs (e.g. phenothiazine, rifampicin and the diagnostic compound bromsulfophthalein). 1. Mucous glands within the ureters and renal pelvis result in the characteristic appearance and texture of equine urine. Mucus secretion is thought to be an evolutionary adaptation to the presence of the rough/irritant calcium carbonate crystals in the urine. 2. On normal diets, urine is often saturated with calcium carbonate, which precipitates spontaneously and gravitates to the floor while urine is held within the bladder. A sample obtained at the onset of urination may be much less turbid than one passed at the end of the stream. Therefore, naturally voided urine and urine collected by catheter frequently appears to vary in its turbidity depending on the part of the bladder being drained at the time. Thick sediment is obtained from the floor while the supernatant is almost clear. Sediment will usually be noted if the container is left undisturbed for a short time. Normal horse urine will foam on agitation due to the natural protein content. 1. The specific gravity of normal equine urine varies between 1.020 and 1.050. 2. Dehydration results in a more concentrated urine (SG over 1.035–1.055). 3. Pre-renal azotemia would be indicated by a high SG and elevated urea/creatinine concentrations. 4. The presence of dilute urine (SG of 1.005–1.020) in an azotemic (elevated creatinine and urea concentrations) or dehydrated horse is indicative of renal azotemia (tubular dysfunction). 5. Fluid therapy in a dehydrated pre-renal azotemia case would result in restoration of the normal concentrations of these metabolites. By contrast, a renal azotemia case would simply produce more urine of an equally dilute nature without normalization of the creatinine and urea concentrations. In acute renal failure, fluid therapy would not normally induce urination within 6 h of the initiation of fluid therapy. A 24 h water deprivation test ( Box 12.3, q.v.) may be necessary to assess renal concentrating ability. However, it is imperative that the horse is carefully monitored during the test to avoid dangerous dehydration. Where dehydration is a real or potential hazard, urine-concentrating ability can be measured following the administration of exogenous antidiuretic hormone. It should be noted that renal medullary washout is due to excessive drinking in the absence of pathology and follows the loss of osmotic gradient within the renal tubules. In this case it is possibly better to perform a partial water deprivation test. The partial water deprivation test is performed by restricting water intake to 40–45 mL/kg/day for several days; water should be offered in small volumes frequently through the day. This will usually restore the gradient, the urine SG will rise to >1.025 and the associated polydipsia will usually resolve. An increase in SG >1.025 suggests psychogenic polydipsia (q.v.) while failure to concentrate >1.025 suggests diabetes insipidus (q.v.). Acidic urine can be established by alteration of the acid-base balance of the diet (dietary cation–anion balance/DCAB). It is calculated using the equation: It is important to realize that erythrocytes may hemolyze if the urine sample is either stood for any length of time or if the sample is agitated roughly. Careful collection, prompt cooling of the sample and timely analysis are, therefore, recommended. Collection of free-flow samples during micturition can give a misleading result and so it is sometimes helpful to obtain the whole urine production or sequential samples so that analysis can provide useful information. Catheterization of the bladder of mares or geldings is practical but again the sample so obtained may be misleading. In general it is wise to collect several samples unless a diagnosis can be established definitively from a single one. A positive “hemoglobin” test may also arise if myoglobin is present, but it cannot identify myoglobin specifically. Differentiation between myoglobin and hemoglobin can be made using spectrophotometric methods and/or the ammonium sulfate precipitation test (Blondheim test *, Box 12.4) (q.v.). Unfortunately this test has largely been ignored but it is a simple and practical way of separating out the two major causes of pigmenturia. A positive glucose test reflects either a reduction in tubular resorption (often termed “a reduction in renal threshold”) or an abnormal blood glucose concentration (often expressed as “exceeding the renal threshold”). Diabetes mellitus (q.v.) is extremely rare in horses but glycosuria is a common feature of equine Cushing’s disease (q.v.); lesser concentrations may simply reflect renal failure. Horses with renal compromise have values >25 but urinary GGT values fall once the acute insult has ceased, despite persistent tubular dysfunction. The value of this assay in progressive or chronic renal failure is therefore doubtful. Creatinine clearance is a useful standard against which the clearance of an electrolyte may be compared in health or disease. The fractional excretion (FE) of an electrolyte is defined as the per cent ratio of its clearance to the clearance of endogenous creatinine. In normal homeostatic balance, FE values vary with dietary and water intake variations, but usually fall within a definable range. With a loss of tubular resorption the excretion of an electrolyte is often increased and its FE rises above the normal range. The FE is derived as follows: divided by which can be simplified to: The FE of an electrolyte is therefore calculated from urinary and plasma concentrations of the electrolyte and creatinine. The FE ranges for healthy horses are shown in Table 12.3. 1. Clearance is influenced by dietary, hydration and endocrine factors. 2. In health, urinary concentrations of electrolytes and their rates of excretion vary between horses and within the same individual through the day. 3. Measurements in horses receiving IV fluids will be spurious, and electrolytes trapped in urinary crystals (calcium and occasionally phosphorus) are not measurable. Normal colt foals will void a normal stream of urine within 4 h of birth while the first urination of filly foals is often delayed up to 6 h. This has implications for the detection of patency of the urinary tract (q.v.). Mature renal function is not achieved for several weeks after birth. Foals often have a transient proteinuria for the first 2–3 days as a result of filtration of small molecular weight proteins absorbed with colostral protein. The high dietary fluid intake results in acidic urine of low specific gravity (1.001–1.004), low osmolarity and high volume (148 mL/kg/day, i.e. up to 6–7L/day). A few epithelial cells and calcium oxalate crystals may be present in normal foal urine. However, the presence of erythrocytes, leukocytes, casts, hemoglobin or myoglobin in foal urine is always abnormal. Urinalysis in normal neonatal foals is significantly different from adult horses: 1. Normal foals frequently show a marked proteinuria for 1–2 days after birth. 2. By nature of their milk diet, foals have a high water intake (approximately 250 mL/kg/day compared with the adult water intake of 50 mL/kg/day). After day 2, urine is hyposthenuric (specific gravity 1.002–1.006) and remains so for several months. 3. Urine pH is neutral to acidic. 4. Urinary enzyme activity and sodium and chloride clearances may be greater than adult values. The age of onset of relevant clinical signs is dependent on the degree of renal pathology and the extent of loss of function. In some cases the conditions are fully compatible with life and are only detected incidentally at post mortem examination at a later age; in others clinical signs are delayed until 6–18 mo although some evidence can usually be found before that.
The urinary system
INTRODUCTION
NORMAL MICTURITION
GENERAL PRINCIPLES OF THE DIAGNOSIS OF URINARY TRACT DISEASE
RADIOGRAPHY
HEMATOLOGY AND BIOCHEMISTRY
URINALYSIS
Visual inspection
Parameter
Normal content
Disease
Hematuria
None
Hematuria reflects inflammation, trauma, neoplasia or coagulopathy in urinary tract. Trace associated with catheterization
Leukocytes
None
Large numbers associated with inflammation of tract (pyuria)
Transitional
Few
Large numbers reflect inflammation, trauma, neoplasia
Bacteria
None
Moderate to heavy Gram smears/cultures reflect pyelonephritis or cystitis
Crystals
Usually calcium carbonate; occasional triple phosphate; occasional calcium oxalate
Large numbers of triple phosphate indicate infection of tract. Large numbers of calcium oxalate are abnormal, but of uncertain significance
Casts
No cellular casts. Occasional hyaline (mucoprotein) casts
Cellular casts reflect tubular damage in association with protein exudation or leakage
Alterations in appearance
Routine urinalysis
Specific gravity
pH
Hemoglobin
Glucose
URINARY ENZYMES
FRACTIONAL ELECTROLYTE EXCRETION
DISORDERS OF THE EQUINE URINARY TRACT
URINARY FUNCTION IN NEONATAL FOALS
Special considerations of renal function in the foal
Urinalysis in foals
CONGENITAL ABNORMALITIES
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The urinary system
Only gold members can continue reading. Log In or Register to continue