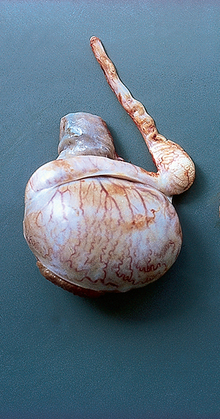
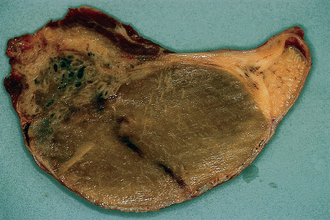
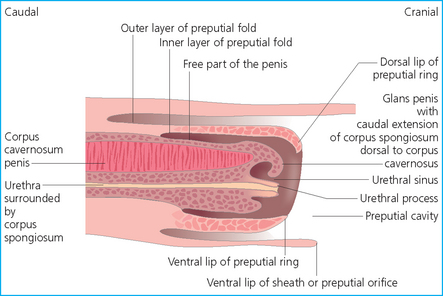
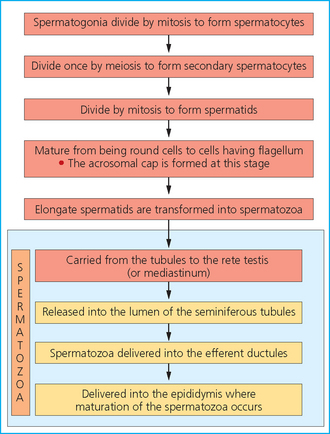
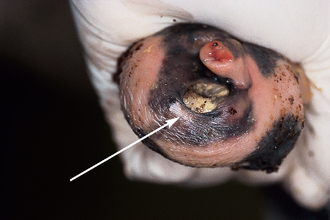
Chapter 4 A complete understanding of the normal anatomy and physiology of the stallion is required in order to evaluate accurately and completely a stallion’s ability to be a successful breeding animal.1–4 The reproductive anatomy of the male horse includes: • The testicles and associated ducts. There are two testicles, located in the scrotum. There are two epididymides and spermatic cords, two vas deferens and two ampullae, which empty into the pelvic urethra. • The accessory sex glands. These include the prostate, the seminal vesicles and the bulbourethral glands. • The external genitalia (Fig. 4.1), comprising the penis and the prepuce. The testicles are located in the scrotum between the hind legs. They lie in a horizontal plane with the long axis of the body and are oblong in shape (Fig. 4.2). They are quite resilient to palpation, resembling a new tennis ball in character. The testicles are the site of spermatogenesis and the production of the hormones testosterone and dihydrotestosterone. They are surrounded by two connective tissue tunics that are loosely apposed to each other (Fig. 4.3; see also Fig. 4.4): Fig. 4.3 The testicular tunics. The tunica vaginalis is an extension of the peritoneum which lines the scrotum. The tunica albuginea is a thick fibrous coat surrounding the testicle. A thin layer of fluid fills the vaginal cavity to allow the testicles to move smoothly in the scrotum. • The tunica albuginea is directly associated with the testicles and is the thicker of the two structures. • The tunica vaginalis is the thinner of the structures and lies outside the tunica albuginea. • The seminiferous tubules, which are lined with germ cells. • Sertoli (or nurse or sustentacular) cells.5,6 These cells have gap junctions between them, which provide a barrier, known as the blood–testes barrier, between the systemic immune system and the germ cells. The horse’s immune system is never exposed to the developing spermatocytes or spermatids and would therefore see them as foreign cells and destroy them if it was not for the protection of the Sertoli cells. Disruption of this protective barrier may result in subfertility or infertility. The Sertoli cells produce proteins that facilitate sperm production and carry nutrients to the sperm cells. • Muscle (myoid) cells and fibroblasts line the tubules, which facilitate movement of spermatozoa and fluids along the tubules. • The interstitium and Leydig cells, which are in the area between the tubules.7 The interstitial tissue is composed of connective tissue, blood and lymphatic vessels and nerves. Testosterone is produced by the Leydig cells and is one of several hormones that facilitate sperm production, as well as supporting the male characteristics of libido, behavior, and masculine appearance. • The seminiferous tubules empty into the straight tubules, which empty into the rete testis, which empties into the efferent ductules and on into the epididymis (Fig. 4.5). From the epididymis the sperm and associated fluids empty into the vas deferens. Fig. 4.5 The tubular system of the testicle. Spermatozoa leave the tubules, move through the straight tubules and empty into the rete testis, then to the efferent ductules which in turn empty into the head of the epididymis. The spermatozoa mature progressively as they pass through the epididymis and are finally stored in the epididymal tail until ejaculation. The stallion is a seasonal breeder and thus the size and nature of the testicles change with the season.1–4 There are also changes associated with age. • During the breeding season, there are more Leydig cells, more Sertoli cells and more spermatozoa per gram of testis. • As the stallion matures, the Leydig cells change from postpubertal to adult-type cells, which produce significantly more testosterone. • The number of Sertoli cells per gram of testis decreases with increasing age. This change is thought to be associated with the decline in fertility associated with old age in stallions and is known as testicular degeneration. • The skin. This has minimal underlying fat and has an abundant supply of sweat glands, which are important in the thermoregulation of the testicles. • The tunica dartos. This comprises smooth muscle fibers and connective tissue. In warm weather, the muscle cells within the tunica dartos relax and allow the testicles to drop away from the body wall; in cold weather, the dartos muscle contracts, helping to pull the testicles closer to the body. • The scrotal fascia. This allows the testicles to move freely within the scrotum. • The common vaginal tunic. Two layers of connective tissue, called tunics, surround the testicles themselves. The outer tunic, the tunica vaginalis or common vaginal tunic, is considered by some to be part of the scrotum and by others to be part of the testicles themselves. The tunica vaginalis is an invagination of the peritoneal lining and passes through the inguinal rings when testicular descent occurs. The tunica albuginea closely adheres to the testicle whereas the tunica vaginalis is separated from the inner tunic by a very thin layer of fluid. This fluid facilitates the movement of the testes in the scrotum. The spermatic cord runs from the internal inguinal ring to the base of the testicles in the scrotum (Fig. 4.6). It consists of: Fig. 4.6 The spermatic cord. This comprises the vas deferens and the pampiniform plexus. The pampiniform plexus contains the testicular vein surrounded by the tightly coiled testicular artery. This arrangement allows for heating and cooling of blood entering and leaving the testicle. • The testicular artery, vein and nerve. The testicular artery is a tightly coiled and convoluted structure, which is important in heating and cooling the blood both entering and leaving the testicles. The testicular vein is intimately associated with the testicular artery. The convoluted testicular artery and vein together are termed the pampiniform plexus. • The cremaster muscle, which is on the lateral aspect of the spermatic cord. It works to raise and lower the testicles during changes in temperature and provides a means of support for the testicles in the scrotum. • In warm weather, the many sweat glands in the scrotal skin provide for evaporative heat loss. The dartos and cremaster muscles relax to allow the testicles to hang further away from the body wall, moving them further from the higher core temperature of the abdominal cavity. This relaxation also allows the spermatic cord to stretch out, resulting in a longer time for blood to pass through the pampiniform plexus, thereby allowing more cooling of the blood to occur. • In the winter, there is no sweating on the scrotal skin and the tunica dartos and cremaster muscles contract. This contraction pulls the testicles closer to the body wall (exposing the testicles to a higher core body temperature). This action effectively shortens the spermatic cord and pampiniform plexus, thereby decreasing the transit time of the blood through the cord and so reduces the alterations in the temperature of the blood as it passes through the cord. Maturation and storage of spermatozoa occur in the epididymis. The epididymis is divided into three sections (Fig. 4.7): Fig. 4.7 The epididymis. This comprises three portions. The caput (head) is bound firmly to the testis, where spermatozoal maturation takes place. The corpus (body) is less closely attached to the testis and leaves a small pocket (the epididymal sinus). The cauda (tail) is attached to the caudal extremity of the testis by the proper ligament of the testis and continues as the vas deferens. Spermatozoa are stored in the cauda (tail) prior to ejaculation. • The head (caput epididymis) is an extension of the efferent ductules of the testicle. The head is rather flat in shape and is closely adherent to the testicle itself. It takes a 180° turn as it courses caudally along the testicle and turns into the corpus epididymis. • The body (corpus epididymis) is tubular and is loosely attached to the dorsal surface of the testicle. • The tail (cauda epididymis) is circular to ovoid in shape and is also loosely attached to the testicle. It terminates in the vas deferens, which takes a 120° turn dorsally as it courses up the spermatic cord. The accessory sex glands of the stallion include (Fig. 4.8): Fig. 4.8 Accessory glands of the stallion. They include paired ampullae, paired seminal vesicles, paired prostate lobes and an adjoining isthmus with paired bulbourethral glands. • The prostate. This comprises two portions: a bi-lobed structure surrounding the urethra and a connecting structure, called the isthmus, between the two lobes. The prostatic lobes are approximately 7 × 4 × 1 cm in size; the isthmus is about 3 cm long. • The seminal vesicles. These are a paired gland comprising vesicular sacs which are filled with the gelatinous fraction of the ejaculate. The seminal vesicles are approximately 15 cm long and 5 cm wide. They empty via a single duct at the seminal colliculus on the dorsal aspect of the pelvic urethra. • The bulbourethral glands. This paired set of glands, at the level of the ischial arch, produce secretions for the maintenance of sperm longevity in the female tract. The urethra is a muscular tube extending from the bladder to the free end of the penis. The pelvic urethra is surrounded by a thick layer of skeletal muscle, which contracts during ejaculation, resulting in the forceful expulsion of semen through the genitourinary tract. The corpus spongiosum penis, part of the erectile tissue of the penis, surrounds the urethra. The distal end of the urethra terminates in the urethral process. • A diverticulum (fossa glandis) surrounds the urethra. • Dirt, debris and secretions will often fill this groove (Fig. 4.9). This accumulation of debris (commonly known as the bean) may cause pain or difficulty in association with ejaculation. Fig. 4.9 Photograph of the end of a stallion’s penis showing the urethral opening surrounded by the urethral fossa and the prominent dorsal urethral sinus. The fossa in this case has a large smegma bean inside it (arrow). • There is also a urethral sinus dorsal to the fossa glandis. • This sinus may harbor infectious organisms, such as that causing contagious equine metritis. The penis of the stallion is musculocavernous in nature. It is composed of three general areas (Fig. 4.10): Fig. 4.10 The penis. (a) The glans free end of the penis comprises the corona radiata, the urethral process and the urethral diverticulum. (b) The corpus cavernosus penis (1) is the major component of the shaft of the penis and fills with blood during erection, resulting in penile engorgement; the corpus spongiosum penis (2) surrounds the urethra (3) and aids in muscular contraction of the urethra during emission. The base of the root of the penis attaches to the pelvis. • The base or root. This is attached to the ischium by the paired crural muscles, the paired ischiocavernosus muscles and a pair of suspensory ligaments. There are paired retractor penis muscles, which relax to allow the penis to be extended and contract to pull the penis back into the prepuce. The urethra runs through the center of the penis and is surrounded by the corpus spongiosum penis. • The corpus spongiosum penis contains some erectile tissue, which becomes engorged when sexual stimulation occurs. • The corpus cavernosum penis surrounds the corpus spongiosum penis. • The corpus spongiosum penis is not surrounded by any connective tissue and continues distally as the glans (free end) of the penis. • The corpus cavernosum penis is an intricately sinusoidal structure, which becomes engorged with blood during sexual arousal. A connective tissue covering called the tunica albuginea surrounds the corpus cavernosum penis. • The glans (free end) of the penis will increase 300–400% in size with engorgement and ejaculation. There are many nerve endings in the glans penis. There is a prominent rim of tissue on the glans (free end) of the penis called the corona glandis, which becomes firm and prominent with penile engorgement. Engorgement of the penis results in a significant (50%) increase in its length and diameter. • The fetal testicle is suspended from the abdominal wall. • The mesonephric duct, which will become the vas deferens and the epididymis, courses caudally towards the pelvic canal. • The vaginal process invaginates to form the inguinal canal. • At around day 150 of gestation, the epididymis is pulled towards the inguinal canal by the gubernaculum; however, due to the large size of the testicle, it cannot pass through the inguinal ring. • The cauda epididymis continues to enlarge as the fetus grows and this stretches the inguinal ring and canal. • At around day 300 of gestation, the testicle is able to enter the inguinal canal. • Abdominal pressure from fluid and intestinal contents help push the testicle into and through the canal. • The right testicle precedes the left testicle in most cases. • Testicles that descend during this time period are considered to have descended within a normal time frame. • Testicles may descend at 6–24 months of age, although these stallions would be considered to be cryptorchid (also known as false rigs or ridglings). • In cases where descent is delayed, the testicle(s) are in the inguinal canal and it may take extra time for them to reach the external inguinal ring and/or scrotum. Failure of testicular descent (cryptorchidism) may be a result of: • Inappropriate abdominal pressure. • Stretching of the gubernaculum. • Inadequate growth of the gubernaculum and cauda epididymis resulting in insufficient stretching of the inguinal ring and canal. • Displacement of the testicle to a position where it cannot be pulled/pushed into the inguinal canal by natural forces. • Deep palpation and/or ultrasound of the external inguinal canal. • Rectal palpation and ultrasound examination following the vas deferens from its exit in the pelvic urethra forward either into the internal inguinal ring or into the abdominal cavity. • Hormone testing. If there is uncertainty about the presence or absence of an abdominal or inguinal testicle, hormonal testing can provide strong evidence for the presence or absence of testicular tissue14 (see p. 94). Physiologically at puberty, spermatogenesis begins as a result of increased testosterone production. Leydig cells produce increasing amounts of dihydrotestosterone, which is converted to testosterone, providing a very high local concentration of testosterone surrounding the germ cells and their nurturing Sertoli cells. Dihydrotestosterone also stimulates the transformation of the stem cells (spermatogonia) into spermatozoa. Surges of luteinizing hormone occur with greater frequency and amplitude, thereby increasing testosterone concentrations. Prolonged stimulation of the Leydig cells is believed to be required for their maturation.16 It has been suggested that, in the prepubertal stallion, production of estradiol results in a negative feedback on the hypothalamus and its production of gonadotropin-releasing hormone.17,18 When production of gonadotropin-releasing hormone increases in frequency and amplitude at puberty, there is also an increased concentration of estradiol receptors, which results in a change from negative to positive feedback. This results in increased amounts of gonadotropin-releasing hormone, which in turn results in increased luteinizing hormone production. Sustained increases in concentrations of the gonadotropic and steroid hormones result in increased testicular growth and development of spermatogenesis. Spermatogenesis is a complicated process involving communication between the testicles and two parts of the brain: the hypothalamus and the pituitary. Several hormones are involved in the production and maturation of spermatozoa, the male sex drive and copulation (Fig. 4.12).19,20 Fig. 4.12 Hormone production and control in the male horse. GnRH, gonadotropin-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; APB, androgen-binding protein; T4, testosterone; DHT, dihydrotestosterone. • Hormones produced in the hypothalamus flow through the portal vessels to the pituitary gland, specifically the pars distalis. • The hypothalamus produces short pulses of gonadotropin-releasing hormone, which is the driving force for the rest of the hormonal cycle. • Gonadotropin-releasing hormone exerts its effect on the anterior pituitary gland and stimulates the release of two hormones: follicle-stimulating hormone and luteinizing hormone. • Both luteinizing hormone and follicle-stimulating hormone are released in a pulsatile fashion, although, during the breeding season, pulses of luteinizing hormone may occur so close together that they are physiologically indistinguishable. The Leydig cells produce the steroid hormones in the testicle:22 • Luteinizing hormone acts on the Leydig cells to produce testosterone, which is delivered to both the local testicular environment and the systemic circulation. • Testosterone feeds back negatively on the hypothalamus and pituitary gland to curtail their production when its concentrations are elevated and positively when its concentration is low. • Some of the testosterone is converted to estrogen by the Leydig cells and some by the hypothalamus and pituitary. • The Leydig cells probably also produce oxytocin, which stimulates contraction of the seminiferous tubules and promotes transport of the germ cells through the tubules. Spermatogenesis is the process of maturation of the testicular germ cells from stem cells to spermatozoa.24 The maturation process requires both mitotic and meiotic division of the germ cells. Spermatozoa are produced by the progression of germ cells. • The primordial germ cells line the prepubertal testicle (with the Sertoli cells) along the outside of the spermatogenetic tubule. • These primordial germ cells are termed spermatogonia. They are few in number until puberty, when the hormonal axis begins to result in a local increase in testosterone concentration. • At this time the number of spermatogonia increases and the first spermatogenetic wave begins. Spermatogenesis occurs in cycles, or waves. Depending on the location in the seminiferous tubule one may find a different stage of the cycle. In a cross-section of a tubule, 4–5 generations are arranged in definitive cellular associations. There are eight stages of the cycle of the seminiferous epithelium (Fig. 4.13):25 • Stage 1: from the disappearance of mature spermatids lining the tubular lumen to the beginning of spermatid nuclei elongation. • Stage 2: from the beginning to the end of spermatid nucleus elongation. • Stage 3: from the end of spermatid nuclei elongation to the start of the first meiotic division. • Stage 4: from the start of the first meiotic division to the end of the second meiotic division. • Stage 5: from the end of the second meiotic division to the first appearance of type B-2 spermatogonia. • Stage 6: from the first appearance of type B-2 spermatogonia to the onset of migration of elongated spermatids towards the tubular lumen. • Stage 7: from the onset of migration of elongate spermatids towards the lumen to the conclusion of their migration and disappearance of all B-2 spermatogonia. • Stage 8: from elongate spermatids all residing at the tubular lumen and the appearance of preleptotene primary spermatocytes until all spermatids have disappeared from the lumen. There are three phases of spermatogenesis: 1. Spermatocytogenesis: 19.4 days of spermatocytogenesis (mitosis and differentiation of the spermatogonia). 2. Meiosis: 19.4 days of meiosis (first of primary spermatocytes, followed by two divisions of meiosis which produce spermatids). 3. Spermiogenesis: 18.6 days of spermiogenesis (differentiation into fully differentiated spermatids). These spermatids are called spermatozoa once they are released from the seminiferous epithelium into the tubular lumen. Spermatogenesis changes with the season of the year.26,27 • Daily sperm production increases during the breeding season. There is a 20% decrease in the efficiency of sperm production in mature stallions during the nonbreeding season.28 Spermatogenesis is also influenced by the age of the stallion.29,30 • As the stallion matures from a postpubertal to a mature stallion, daily sperm production increases along with testicular size. • In aged stallions (over 13 years of age), efficiency of sperm production decreases by around 35%. Spermatozoa are continuously produced after the onset of puberty.31 There is continuous production of differentiated and committed spermatogonia, which go on to become spermatocytes and then on to spermatids and eventually spermatozoa. Uncommitted spermatogonia are replaced so that the germinal epithelium always has a supply of stem cells from which spermatozoa can be produced. Thermoregulation of the testicles is an important function of the scrotum and spermatic cord.32 • Normal thermoregulation is required for spermatogenesis to proceed in an ordinary fashion. • The scrotum regulates temperature by altering the position of the testicles, either closer to or further away from the body wall, depending on the environmental temperature. The dartos and cremaster muscles contract in cool temperatures and relax in warm temperatures to bring the testicles closer or further from the body wall, respectively. • The scrotum and the pampiniform plexus control testicular temperature through vascular exchange. Erection, emission and ejaculation are complex neurological and physical processes.35,36 • Sensory stimulus of the glans penis and psychological stimulation of the cerebral cortex initiate erection. • Parasympathetic impulses from segments S2–S4 pass to the penis resulting in dilation of the penile arterioles. • There is an increase in the amount of blood entering the corpus cavernosus penis and the corpus spongiosum penis. • The muscles of the penile root simultaneously contract and compress the veins of the penis against the ischial arch; this blocks the venous return from the penis, leading to penile engorgement. Cardiac output also increases, resulting in increased blood flow and pressure in the caudal aorta. • Erection is lost when sympathetic impulses dominate again, resulting in arteriolar contraction, and the penile root muscles relax, resulting in the restoration of normal venous outflow. Ejaculation is the forcible expulsion of fluids and spermatozoa through the urethra. • It is controlled by parasympathetic impulses. • During emission and ejaculation the urethralis and bulbospongiosus muscles contract strongly, resulting in pulsation and deposition of semen into the female tract. • Prostatic fluid is emitted first (pre-ejaculatory fluid). • Then the sperm-rich fraction is produced in 3–6 jets. The sperm-rich fraction also contains prostatic, bulbourethral and epididymal fluids. • The last portion of the ejaculate comes from the vesicular glands and is gelatinous in nature (the so-called gel fraction). • Daily sperm output can be determined by collecting the stallion daily for 5–7 days. At this time, the extragonadal sperm reserves are stabilized. When the stallion is collected on day 8, an accurate assessment of daily sperm output can be made. It can also be estimated by using testicular dimension measurement (see below). • When stallions are collected daily, the daily sperm output will vary from 3.2 to 6.6 billion spermatozoa. • The number of spermatozoa per milliliter of semen differs between first and second ejaculates, collected 1 hour apart, by approximately 55%.37 There is a minimal reduction in gel-free semen volume for the second ejaculate. • In stallions collected once versus twice daily, the sperm output is similar, regardless of the frequency of ejaculation. Therefore, a defined number of sperm is available on a daily basis for ejaculation and once the daily sperm output is reached there is no benefit of collecting twice daily for artificial insemination programs. • In stallions being utilized in natural service programs, adequate time between services must be provided such that extragonadal sperm reserves are replenished. The primary factors that influence daily sperm output include: Other, secondary, factors that may affect sperm output include: There is a significant effect of season on daily sperm production.39 • The normal breeding season in the northern hemisphere is May–August. In the southern hemisphere the season extends from September to December. • Semen volume is approximately 40% higher during the breeding season than during winter. The average number of spermatozoa per ejaculate is about 50% higher during the breeding season than during the nonbreeding season. • If stallions are exposed to decreasing day length in autumn and then stimulated by an artificial photoperiod (16 light hours, 8 dark hours), the seasonal increase in sperm production can be stimulated earlier in the breeding season such that maximal sperm production in the northern hemisphere will occur in April rather than in June.40 However, in these stallions, sexual recrudescence will occur earlier in the breeding season. Therefore, if the majority of mares are to breed in February–May, placing stallions under artificial lighting will be of benefit. However, if the majority of mares will be bred during May–July, artificial lighting is not recommended. • The more mature a stallion is, the larger his epididymal sperm reserves will be.41 • However, an aged stallion may have fibrosis of the epididymis resulting in decreased epididymal sperm reserves. • Younger stallions produce less total semen volume, less gel-free volume and less total spermatozoa per ejaculate than do mature stallions (>6 years). • There is no difference between 6-year-old stallions and 16-year-old stallions. It is evident, therefore, that maturation of the stallion is complete by 6 years of age. Frequency of ejaculation will affect total volume and number of spermatozoa per ejaculate until the daily sperm output is reached (Table 4.1).1–4 Table 4.1 Effect of frequency of ejaculation on total semen volume and number of spermatozoa per ejaculate (estimated for a mature stallion) • The more frequently an individual stallion is collected the lower will be his semen volume. Once the daily sperm output is attained, a similar frequency of ejaculation will not result in further decreases in semen volume. • In stallions that are collected at least every other day, the number of spermatozoa per ejaculate decreases as the frequency of ejaculation increases. • When stallions are collected every other day, all the spermatozoa being produced are being ejaculated. • Therefore collecting any more often than every other day will result in decreased semen concentration per ejaculate. • Young stallions that are collected twice daily may have inadequate sperm production for normal fertility (especially when used for natural service). Sperm stored in the ampulla, vas deferens and cauda epididymis can be released during ejaculation. • Sperm located in the caput and corpus of the epididymis are not available for ejaculation until maturation and transit of the sperm are completed. • The number of sperm available for ejaculation depends on the size of the epididymal sperm reserves and the frequency of and interval between ejaculations.45,46 • Approximately 62% of the sperm available for ejaculation is stored in the tail of the epididymis. • The larger the testicles, the greater the potential for increased sperm production. • Although testicular size is an important factor, it is also important that the testicular tissue that is present is normal and completely functional for sperm output to be maximal. • There are pathological reasons for enlargement of testicles and in this circumstance the output will be significantly impaired. Testicular measurements can be made using calipers (Fig. 4.14) or by using an ultrasonographic measurement system. • When measuring the testicles, they should be pressed downwards fully into the scrotum with one hand, while the other hand performs the measurements. • Measurement of testicular depth, width and length should be repeated at least three times to be sure that an accurate overall measurement is made. • If total scrotal width is used as an indicator of daily sperm output, again at least three measurements should be made to reduce the degree of error. Testicular size, daily sperm production and extragonadal sperm reserves continue to increase for several years following puberty. • Testicular size is highly heritable in other species and is likely heritable also in the stallion. • Stallions with a total scrotal width of less than 80 mm should not be used in breeding programs as they may pass on the genetic characteristics of hypoplasia. Additionally, stallions with low total scrotal width are poor sperm producers and will likely suffer fertility problems. • Forty percent of the variation in testicular size is accounted for by difference in age. • For the young stallion, collection or service once daily throughout the breeding season should be possible if all physical parameters are normal. • In the older stallion, collection or service is possible up to three times daily, if libido will allow it. • Evaluation of the physical status of the entire animal. • Specific examination of the reproductive tract. • Determination of the normality of the spermiogram. • Calculation of the number of mares that can successfully be bred during the ensuing breeding season, which includes determination of daily sperm output via evaluation of testicular function. Evaluation may need to be performed: • As part of a pre- or postpurchase examination. • Prior to the breeding season. • If fertility should decline. • If breeding behavior changes. The Society of Theriogenology has established guidelines for evaluating fertility potential of stallions.47 The results of the breeding soundness examination should be recorded carefully, preferably using a standardized form.48 These results are often required for legal and medical reasons and errors and omissions should be avoided. The breeding soundness examination is used to make recommendations on the number of mares that a stallion may mate successfully each season. • Significant fluctuations occur in the health status of a horse. • Many causes of infertility in stallions are temporary (e.g. illness, injury, and reduced libido) so a breeding soundness examination performed when the stallion is not in his normal state could easily lead to his being condemned unjustifiably. The standard examination comprises the following: 1. The stallion should always be positively identified from documents provided. The identification should include: • Identifying marks: lip tattoo, hide brands (hot and freeze brands), color markings and hair whorls. 2. A complete history that details medical problems, injuries, drug therapy the stallion has been receiving over the past 6 months, and vaccination status needs to be taken. It should also include: • The dates of the athletic performance career (beginning and end). • The present use of the stallion (if used other than for breeding). • Previous breeding performance, including first cycle conception rates. • The pregnancy rate for the previous seasons and the current year. • Libido, mating behavior record. • Abnormalities encountered during breeding. • Results of previous fertility examinations and past breeding management should be evaluated and compared with the present findings, especially if the stallion has decreased fertility. • Any positive or negative experiences should be noted, especially if the stallion has a behavioral problem. • Mismanagement, inadequate nutrition, or a concurrent medical disorder may lead secondarily to deterioration in semen quality. 3. Physical examination of the whole horse to assess general health, including blood and serological testing for infectious or other disorders that may be directly involved in breeding (e.g. dourine, equine viral arteritis): • The examination must be thorough and the results properly recorded: reports and results are often required for sale purposes, for obtaining fertility insurance or for making recommendations on breeding management. • Particular attention should be directed to auscultation of the heart, evaluation of the eyes and the ability of the stallion to move freely. • Observation of the stallion moving in a small paddock may help to identify any musculoskeletal problems such as lameness due to chronic degenerative joint disease, laminitis or back disorders. • Potentially heritable traits such as cryptorchidism, brachygnathia, or cervical vertebral instability (‘Wobbler’) syndrome need to be recorded. • Rectal examinations are not routinely performed due to the inherent risks involved to both the examiner and the stallion. 4. Detailed evaluation of external genitalia (and possibly the accessory sex glands): • Examination of the reproductive tract of the stallion is conducted primarily to evaluate fertility potential and to diagnose gross abnormalities of the external genitalia. • The scrotum and its contents should be evaluated for symmetry and size: 5. Evaluation of libido and sexual behavior requires observation of mating behavior when presented with an estrus mare. 6. Evaluation of mating ability requires direct observation of mating. 7. Semen quality requires collection of a truly representative semen sample: • Spermatozoal and semen characteristics and the microbial flora of semen. • Ejaculated semen is composed of heterogeneous subpopulations of sperm cells, making an accurate diagnosis of dysfunctional sperm very challenging. • Some stallions, under good breeding management systems, that have normal to moderately low numbers of morphologically normal, motile sperm in their ejaculates, either fail to have normal conception rates or have decreased conception rates. • If pus, bacteria or blood are present in the ejaculate, or abnormalities such as azoospermia or cryptorchidism are noted, a rectal examination should be performed to assess the internal genitalia and inguinal rings. • Supporting diagnostic tests (such as radiography, endoscopy, and blood sample analysis) should be performed if there is any doubt about the findings of the physical examination. • Additional methods for identifying possible causes of the subfertility are currently available in specialized laboratories. Technological advances in semen preservation have changed breeding management strategies. • Breeding soundness examinations may be performed on stallions that are to breed only 10–20 mares, but semen collected from that particular stallion must be cooled and shipped.
THE STALLION
ANATOMY
The testicles
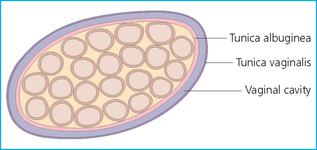
The scrotum
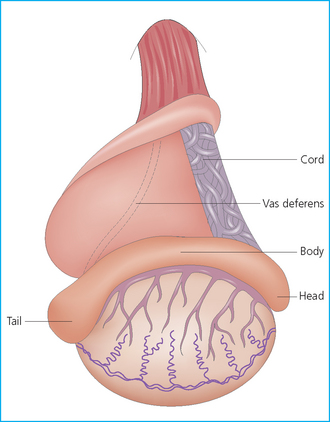
The spermatic cord
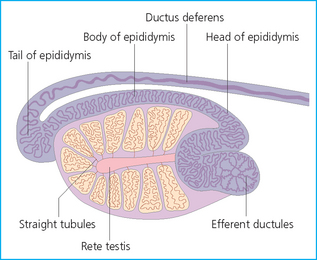
The epididymis
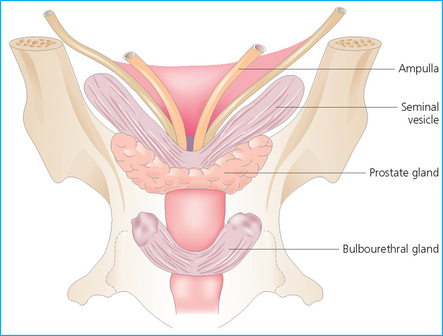
The accessory sex glands
The urethra
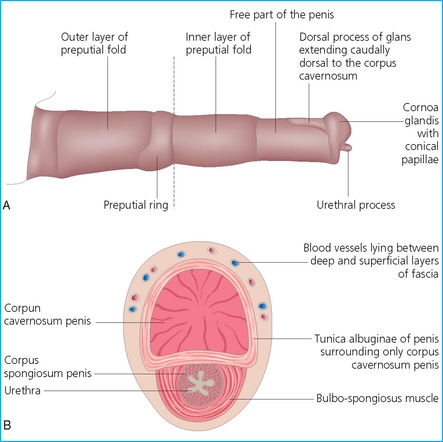
The penis
DESCENT OF THE TESTICLES11,12
Failure of normal testicular descent (cryptorchidism)
PUBERTY
PHYSIOLOGY OF SPERM PRODUCTION
Hormonal control
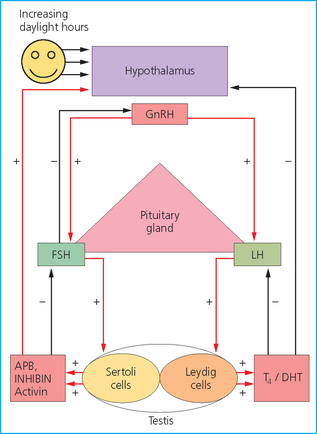
 Follicle-stimulating hormone exerts positive feedback on the testicles by acting on the Sertoli cells and induces release of androgen-binding protein, inhibin and activin.21
Follicle-stimulating hormone exerts positive feedback on the testicles by acting on the Sertoli cells and induces release of androgen-binding protein, inhibin and activin.21
 Follicle-stimulating hormone is not completely dependent on gonadotropin-releasing hormone secretion in the male. Inhibin and activin are released into the general circulation and feed back on the hypothalamus and pituitary to inhibit and promote, respectively, the release of follicle-stimulating hormone.
Follicle-stimulating hormone is not completely dependent on gonadotropin-releasing hormone secretion in the male. Inhibin and activin are released into the general circulation and feed back on the hypothalamus and pituitary to inhibit and promote, respectively, the release of follicle-stimulating hormone.
 Androgen-binding protein binds to both dihydrotestosterone and testosterone and provides a high concentration of both of these hormones locally around the germ cells.
Androgen-binding protein binds to both dihydrotestosterone and testosterone and provides a high concentration of both of these hormones locally around the germ cells.
Spermatogenesis
Thermoregulation
Erection and ejaculation
Erection
Ejaculation
Daily sperm output
Factors affecting sperm production/output
Season of the year
Age of the stallion
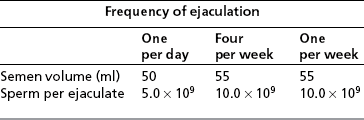
Frequency of ejaculation
Testicular size and consistency
BREEDING SOUNDNESS EXAMINATION
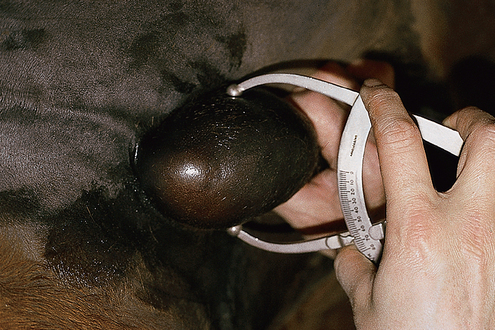
 Total scrotal width or testicular volumes are measured using calipers or testicular ultrasonography, respectively.
Total scrotal width or testicular volumes are measured using calipers or testicular ultrasonography, respectively.
 The size of the testicles is directly related to the male’s daily sperm output and the number of mares he is able to breed during one breeding season (see p. 52).
The size of the testicles is directly related to the male’s daily sperm output and the number of mares he is able to breed during one breeding season (see p. 52).
 Testicular consistency is assessed. The testicles and epididymides are palpated for tone, symmetry, and the presence of any abnormally firm or soft areas (see p. 55).
Testicular consistency is assessed. The testicles and epididymides are palpated for tone, symmetry, and the presence of any abnormally firm or soft areas (see p. 55).
 Both testicles should be present in the scrotum.
Both testicles should be present in the scrotum.
 The spermatic cords are palpated for the presence of the vas deferens, sperm granuloma, varicocele (dilation of the blood vessels), excessive fat or masses in the cord.
The spermatic cords are palpated for the presence of the vas deferens, sperm granuloma, varicocele (dilation of the blood vessels), excessive fat or masses in the cord.
THE STALLION