Chapter 17 CONGENITAL AND DEVELOPMENTAL PROBLEMS Cervical vertebral malformation Occipitoatlantoaxial malformation Equine degenerative myeloencephalopathy Hypoxic-ischemic encephalopathy (neonatal maladjustment syndrome) INFLAMMATORY AND TOXICO-INFECTIOUS DISEASES Bacterial meningoencephalitis/brain abscess Western, eastern and Venezuelan encephalomyelitis West Nile virus myeloencephalomyelitis Equine herpesvirus 1 (EHV-1) myeloencephalopathy Leukoencephalomalacia (moldy corn poisoning, mycotoxic encephalomalacia) Yellow star thistle (nigropallidal leukoencephalomalacia) Rye grass and dallis grass tremorogenic mycotoxicoses (staggers) POST-ANESTHETIC CENTRAL NERVOUS SYSTEM DISORDERS CONDITIONS OF THE LOWER MOTOR NEURON UNIT Hyperkalemic periodic paralysis Shivering, polysaccharide storage myopathy and stiff horse syndrome Vestibular syndrome (head tilt) Suprascapular nerve paralysis (sweeny) Polyneuritis equi (cauda equina neuritis) With the advent of equine protozoal myeloencephalitis (q.v.) and West Nile virus (q.v.), neurologic diseases of horses have become increasingly significant worldwide. Since the laboratory diagnosis of those two diseases is not straightforward, and other diseases need disparate treatments and offer a different prognosis, there is increasing pressure on clinicians to make the correct clinical diagnosis. Cerebellar abiotrophy is suspected to be an inherited disorder with an 8% incidence in some Arabian family lines. It is also considered an inherited disorder in Oldenburg light-breed horses and in Eriskay and Gotland ponies. Either gender can be affected. The diagnosis is based on breed, history and clinical signs. The signs may be present at birth, but can be difficult to recognize in foals <1 mo of age. The clinical signs are characterized by jerky head movements and hypermetria/dysmetria. Affected foals assume a wide-based stance at rest and may fall over backwards if asked to back up or elevate the head. As the foal ages, it will appear strong but ataxic and continue to exhibit a rhythmic head bobbing or head tremors, characteristic of cerebellar disease (q.v.). Blindness and gait paresis are not features of this disease. Once other causes for seizures have been ruled out, a diagnosis of idiopathic epilepsy can be given. Although the disease is self-limiting with age, seizures must be kept under control ( Table 17.1) to avoid permanent brain damage or head trauma. Initial anticonvulsant therapy with diazepam (5–20 mg IV) repeated two or three times will usually control the seizures. For maintenance anticonvulsant therapy, affected foals should be kept on phenobarbital (100–500 mg PO s.i.d. or b.i.d.) for a minimum of 10–14 days, or as long as is needed to avoid recurrence of seizure activity. Treatment with phenobarbital should be dose adjusted so that seizures can be controlled but the foal not overly sedated. Monitoring blood levels of the drug is advised and the non-toxic therapeutic range is approximately 5–30 μg/mL. The lowest dose that controls seizures should be used, as phenobarbital may sedate the foal and discourage nursing. The dose must be tapered down over 2 weeks before being discontinued, as abrupt discontinuation may precipitate seizures. Table 17.1 Guidelines for selected anticonvulsant drugs used to treat seizures in adult horses and foals Pediatric epilepsy generally subsides as the foal ages, as long as seizure-related brain damage has not occurred. If blindness persists in the postictal phase, vision usually returns within a few days after seizure activity is controlled. The incidence of recurrence after puberty is unknown. The neurologic examination and plain radiographs of the vertebrae (C1–T2), while the horse is standing with the neck in a neutral position, are the most important diagnostic aids. In younger horses plain film radiographs reveal stenosis of the vertebral canal, often at C3–4 or C4–5. A corrected minimum sagittal diameter (the ratio of the absolute minimum sagittal diameter of the vertebral canal to the sagittal width of the vertebral body) of <0.5 is highly suggestive of a compressive lesion at C3–4 or C4–5. EDM is rarely seen in horses ≥4 yr of age. Symmetrical ataxia, hypometria and paresis usually appear in animals ≤6 mo of age. The disease is progressive, but the clinical signs have generally stabilized after the animal reaches 6–12 mo of age. Signs may be more severe in the pelvic than the thoracic limbs. Clinically the disease can be difficult to distinguish from cervical vertebral malformation (q.v.). Intensive supportive care (q.v.) is critical to the survival of a foal with HIE. Therapy for the various manifestations of hypoxia-ischemia involves control of seizures, general cerebral support, correction of metabolic abnormalities, maintenance of normal arterial blood gas values, maintenance of tissue perfusion, maintenance of renal function, treatment of gastrointestinal dysfunction, prevention/recognition/early treatment of secondary infections and general supportive care. It is important that seizures be controlled as cerebral oxygen consumption increases five-fold during a seizure (see Table 17.1). Parenteral antibiotics (2.2 mg/kg ceftiofur IV b.i.d.) are indicated if sepsis is suspected. Nutritional and respiratory support may be necessary. Correction of acid-base, electrolyte and glucose abnormalities is indicated. With intensive supportive care, 75–80% of HIE foals will survive. In survivors, the condition usually stabilizes by 2–3 days of age and improvement is noted by Day 4. The neurologic signs often resolve in the reverse order in which they appeared. Complete recovery may take up to 3 mo. Failure of passive transfer of colostral antibodies concurrent with HIE is associated with a poor prognosis. Antimicrobial therapy should be based on culture and sensitivity along with the capacity of the drug to cross the blood–brain barrier (BBB). The antibacterial agent should be highly lipid soluble, non-ionized and poorly protein bound to optimize penetration. In the early stages, or if microbial culture of CSF or other body fluids is negative, empiric treatment with bactericidal, IV delivered antibiotics is advised. Recommended antibiotics include trimethoprim-potentiated sulfonamides (20–35 mg/kg IV b.i.d. or t.i.d.), or a third generation cephalosporin (ceftiofur 5–10 mg/kg t.i.d.), in combination with benzylpenicillin (20000–50000 IU/kg IV q.i.d.). Aminoglycosides such as gentamicin (6.6 mg/kg IV s.i.d.) could also be given in combination with penicillin, but as meningeal inflammation subsides, they may not penetrate the BBB as effectively. Only the cephalosporins and trimethoprim-potentiated sulfonamides have adequate penetration at all times. Therapy should be prescribed for a minimum of 6–8 wk. Tetracycline does not penetrate the BBB and should be avoided. Additional treatment with anti-inflammatories such as 1.1 mg/kg flunixin PO or IV b.i.d. is also recommended. Anticonvulsant therapy (see Table 17.1) may be necessary. Clinical signs of EE, WE and VE are generally similar and differ only in detail. These diseases frequently affect younger horses (1–2 yr of age), although horses of any age can be affected. A low grade infection may be present, characterized by low grade viremia, fever, lymphopenia and neutropenia. A generalized febrile illness may be observed and can be characterized by anorexia, obtundation, tachycardia, diarrhea (VE), lymphopenia and neutropenia. A few horses may die in this stage of the disease. Myeloencephalomyelitis is classically characterized by clinical signs suggesting diffuse cortical disease. Affected animals may become obtunded, unresponsive or irritable. Head pressing, leaning on walls or fences, compulsive walking, circling, blindness, lack of menace response, cranial deficits, or an unsteady gait may be present. Clinical signs progress within 12–48 h. Death is usually preceded by recumbency, irregular breathing, cardiac arrhythmias, coma and convulsions. Survivors may recover over a period of weeks but may have residual deficits (“dummies”). The West Nile virus (WNV) (q.v.) is a mosquito-borne flavivirus in the Japanese encephalitis complex, and is endemic in Africa and Asia. West Nile virus was first identified in the West Nile district of Uganda in 1937, and has since been found in other parts of Africa, Eastern Europe, West Asia, the Middle East and the USA. It is maintained in cycles involving birds as vertebrate hosts and mosquitoes as vectors. The virus is transmitted by at least 10 genera of mosquitoes and has been identified in more than 100 species of birds, notably the American crow. Antibodies or disease have also been shown in humans and an impressive range of animals, from alligators to bears to horses. Birds appear to be the only animal with sufficient viremia to infect mosquitoes. After entry, the virus remains localized for periods that vary from days to many months, resulting in a large variability in the incubation period from weeks to months. After virus multiplication in the connective tissue and muscle at the site of injury, virus is spread by replication in Schwann cells or by axoplasmic transport (it does not replicate in axoplasm). Early replication occurs in dorsal root ganglia and this correlates with the tingling sensation at the bite or scratch site seen in the prodrome of some human cases. After initial replication in the dorsal root ganglia, the virus disseminates rapidly and selectively in the CNS to infect neuronal cells of the brainstem, hippocampus, the subcortical nuclei, the Purkinje cells of cerebellum and limbic cortex. In the second phase, the virus spreads via the nerves (not blood) to diverse sites such as the eye, salivary gland, papillae of tongue, heart, hair follicles of skin, and some muscles. The clinical course of the disease is related to the dose and site of inoculation (i.e. proximity to the brain) and pathogenicity of the specific virus strain. In horses, natural infection is invariably fatal. The currently marketed inactivated rabies vaccines are thought to be safe and effective. Annual vaccination of horses against rabies is recommended in areas where the disease is endemic. Although the horse is moderately susceptible to rabies, transmission from a rabid equid to a human has not been documented. If an unvaccinated horse is bitten it should not be vaccinated immediately but isolated for 6 mo and vaccinated 1 mo before the end of quarantine. If at any stage exposure to rabies is confirmed the horse should be euthanased immediately. There are no specific treatments for EHV-1. Supportive care is essential. Deep bedding, laxatives, enemas, manual rectal evacuation, urinary catheterization and antimicrobial therapy for secondary infections (bronchopneumonia, cystitis) may be necessary. The use of corticosteroids in the acute phase (dexamethasone, 0.1–0.25 mg/kg b.i.d. IM or IV for 1–3 days) is controversial but could be attempted if bacterial infection of the CNS has been ruled out. Corticosteroids also have a depressant effect on lymphocytes, which may be undesirable in cell-associated viral infections such as EHV-1. Since the CNS lesions appear to be immune mediated, vaccination of horses showing neurologic signs could worsen the disease. There is no definitive ante mortem diagnostic test. Routine CSF analysis is often normal. Serology looking for antibodies is useful to rule out disease but has a low positive predictive value. CSF antibodies may be found in the absence of CNS infection. The combination of clinical signs and a positive response to therapy has been used as a presumptive diagnostic tool. Electromyography can help localize lower motor neuron (gray matter) involvement.
The nervous system
INTRODUCTION
CONGENITAL AND DEVELOPMENTAL PROBLEMS
CEREBELLAR ABIOTROPHY
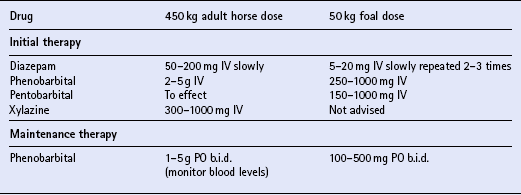
JUVENILE EPILEPSY OF FOALS
CERVICAL VERTEBRAL MALFORMATION
EQUINE DEGENERATIVE MYELOENCEPHALOPATHY
HYPOXIC-ISCHEMIC ENCEPHALOPATHY (NEONATAL MALADJUSTMENT SYNDROME)
INFLAMMATORY AND TOXICO-INFECTIOUS DISEASES
BACTERIAL MENINGOENCEPHALITIS/BRAIN ABSCESS
WESTERN, EASTERN AND VENEZUELAN ENCEPHALOMYELITIS (WE, EE, VE)
WEST NILE VIRUS MYELOENCEPHALOMYELITIS
RABIES
EQUINE HERPESVIRUS 1 (EHV-1) MYELOENCEPHALOPATHY
PROTOZOAN MYELOENCEPHALITIS
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


