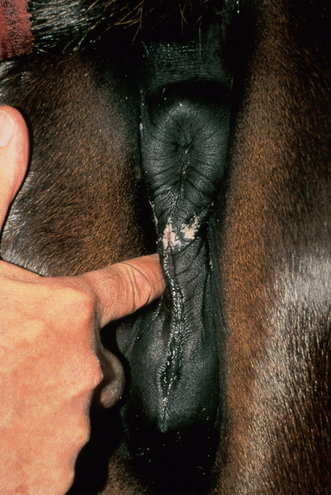
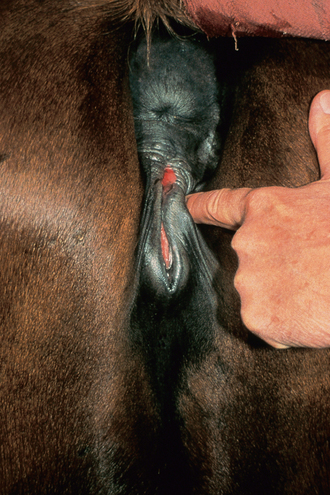
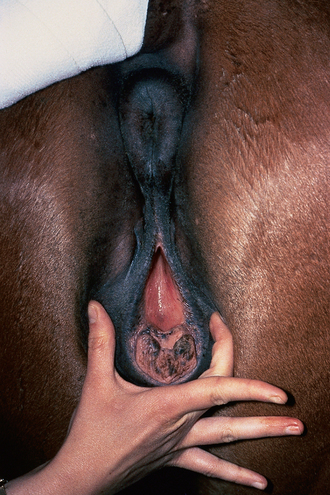
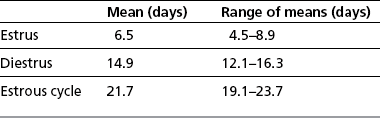
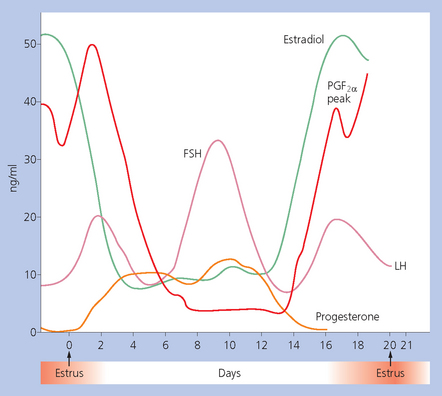
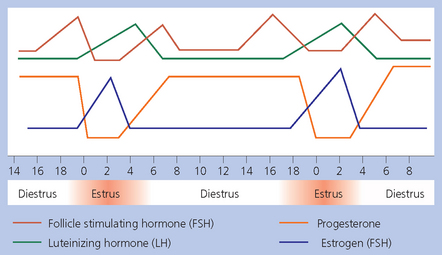
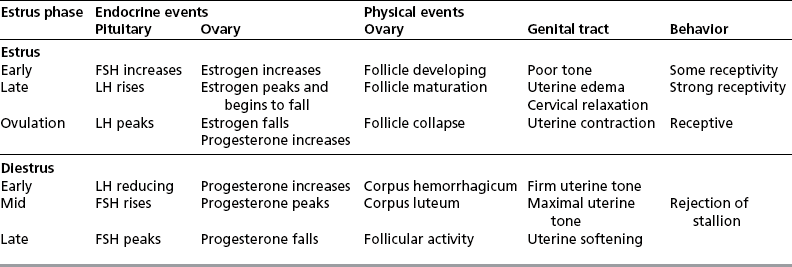
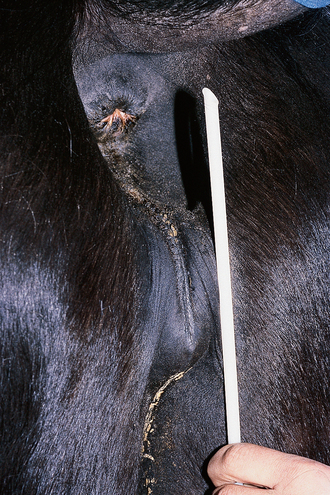
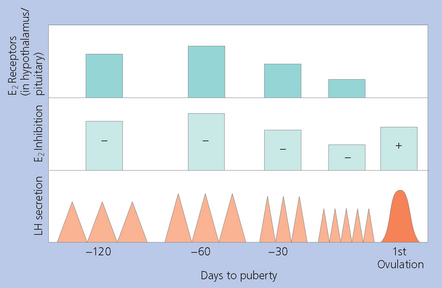
Chapter 5 A thorough understanding of the mare’s reproductive anatomy is needed to differentiate normal from abnormal, and to identify structures during reproductive examinations. In this section important anatomical considerations are reviewed. The main structures of the breeding organs of the mare are shown in Fig. 5.1. Fig. 5.1 Reproductive organs of the mare: (A) left lateral view; (B) viewed from above; (C) detail of the falopian tube region and the relationship of the horn of the uterus to the broad ligament. The perineum is the area that includes the anus, vulva and the adjacent skin (usually hairless) under the tail. The normal conformation of the perineum prevents the ingress of air and bacteria into the genital tract. This area is very important because of its protective role for the genital tract and its implications in some forms of infertility in particular. It is also a common site for injury at parturition. The anatomic arrangement of the perineum, including its length relative to the pelvic bones and its angle relative to the vertical plane, are important features for the fertility of the mare; for example, mares that experience bouts of infertility frequently have a flat-topped croup with a tail setting that is level with the sacral iliac joint and a sunken anus (Fig. 5.2). Alterations of angle and length have been used to determine a Caslick score1 (see p. 185) which is used to provide an index of the need to perform a vulvoplasty operation (Caslick’s operation, see p. 184). The normal/ideal anatomic arrangement is that the vulva should be vertical (or at least less than 10% from the vertical) and that more than 80% of its length (from dorsal commissure top ventral commissure) should lie below the level of the ischial tuberosities (Figs 5.3, 5.4). Variations in either the relative length below the ischium or an increase in the angle of declination (or both) results in a tendency for the vulva to be drawn forwards into the perineum below the anus, especially during later pregnancy (Fig. 5.5). This combination results in an increased chance of aspiration of air into the vagina and a higher Caslick score. Fig. 5.5 An example of very poor vulvar conformation. The upper half of the vulva slopes forward and is almost horizontal. The anus is drawn forward. Three seals protect the genital tract: • The vulvar seal (created by close contact between the vulvar lips or labiae and the skin and muscles of the perineum). • The vestibulovaginal seal (formed by the posterior vagina, the pillars of the hymen and the floor of the pelvic girdle). Any deficiency in one or more of these ‘seals’ has implications for the reproductive efficiency of the mare. During a normal reproductive examination these seals are broken and air allowed into the vagina. The presence of air in the vagina allows bacteria to be carried into the anterior reproductive tract. The presence of the air itself is responsible for a typical inflammatory response with blood vessel engorgement. When any of the three seals are disrupted (either through trauma, faulty acquired or congenitally poor perineal conformation, or poor body condition) air may be intermittently or consistently aspirated into the vagina and the resulting contamination and infection can be a serious cause of infertility. The vulvar and vestibulovaginal seals usually restrict the ingress of clinically significant bacteria. The vagina and uterus should be free of significant infection although some incidental bacteria are commonly found.2 The vulva comprises the two vulvar lips (labiae) (and the clitoris) and is one region of the reproductive tract that is in common with the urinary system. The two vulvar lips meet dorsally at the tightly angled dorsal commissure and ventrally at the more rounded ventral commissure. Just inside the lips themselves lies the junction between the highly glandular skin and the nonglandular mucous membrane of the vestibule (the area of the reproductive tract that lies outside the level of the vestibulovaginal seal/hymen). The mucocutaneous junction is continuous with the clitoral prepuce or fold. The vulvar constrictor muscle continues into the external anal sphincter dorsally. The clitoral retractor muscle lies over the vulvar constrictor muscle ventrally. These muscles are variously responsible for the protective function of the vulva and the winking of the clitoris that occurs at the end of urination and during estrus, and the arrangement allows considerable expansion during delivery of the foal. The erectile clitoris of the mare is held in a protective pouch (the clitoral prepuce) just inside the ventral commissure of the vulva. It can readily be everted by gentle manual pressure applied across the ventral commissure of the vulva (Fig. 5.6). This process reveals the clitoral glans itself, which has a wrinkled appearance in most mares. The clitoris lies in the clitoral fossa created by the clitoral prepuce and it is common to encounter an accumulation of moist smegma in this area. On the crown of the clitoris are three depressions (the clitoral sinuses) with a deep central sinus and two smaller, shallower lateral sinuses. All of these commonly harbor a variable amount of smegma derived from the clitoral sebaceous glands. The clitoral sinuses and fossa have considerable importance in the investigation of venereal disease. Bacteriological swabs are routinely taken from the clitoral sinuses and from the clitoral fossa (see p. 152). The cervix is a muscular structure 5–7.5 cm long and about 2–5 cm in diameter. It is a very important structure for reproductive efficiency. It projects some 2.5–4.0 cm into the anterior vagina. It forms the final barrier to the ingress of contamination and infection. During estrus it dilates to allow the passage of semen into the uterus and at this time it is easily dilated manually. An operator should easily be able to pass one or two fingers through its entirety during estrus. During diestrus and pregnancy the cervix is firm and tightly closed and it is difficult to dilate manually. During parturition it has to dilate very considerably; failure to dilate sufficiently during delivery of the foal at parturition can result in traumatic laceration (see p. 303). The surface of the cervix is a delicate mucous membrane that is easily traumatized by strong chemicals. Adhesions and damage are important causes of infertility (see p. 174). Examination of the cervix usually involves manual palpation per vaginum and visualization using a speculum or endoscope (see p. 136). The uterus is a T-shaped structure. The two uterine horns and body are located entirely within the caudal part of the abdominal cavity. Distension and movement of the intestines and bladder as well as pregnancy influence the position of the reproductive tract.3 The uterine horns range from 20 to 25 cm in length. The uterine body averages 18–20 cm in length. The ovaries can move freely within the abdomen, thus their orientation and location are variable, making it difficult to identify poles and surfaces. The mesovarium attaches at the dorsal aspect of the ovary and extends for a considerable distance over the medial and lateral surfaces. Blood vessels and nerves reach the ovaries through the broad ligaments and enter each ovary at the dorsal convex border and spread over the lateral and medial surfaces. The oviducts are tortuous tubes within the mesosalpinx, with a total length of some 20–30 cm. At ovulation, ova drop into the expansive funnel-shaped infundibulum that covers the ovulation fossa. They travel quickly through the infundibulum to the ampulla where fertilization and early development of an ovum occurs. The fertilized ovum then travels through the narrow isthmus and enters the uterine horn at the uterotubal junction. Unfertilized ova normally are retained for a considerable time in the oviduct before degeneration occurs.4 Unfertilized ova rarely enter the uterus. The onset of puberty usually occurs, and is currently presumed to result, from a combination of endocrinological events within the hypothalamus, the pituitary gland, and the gonads (Fig. 5.7). The onset of puberty is well recognized as a primary reproductive event and occurs at around 12–24 months of age,5 but this can be significantly affected by several factors, including: Fig. 5.7 Model for endocrine control of puberty. A minus sign (−) indicates inhibitory effects of estradiol; a plus sign (+) signifies a positive effect. E2, estradiol; LH, luteinizing hormone. • Age (and timing of birth within the breeding season). • Photoperiod (daylight hours). This is likely to be a significant event in the onset of puberty in horses, but this area has been poorly researched. Prolonged daylight hours result in a delayed onset of puberty; the best stimulus to puberty is the natural fluctuations in daylight hours.6 This contrasts with the effect of photoperiod in mares and stallions of mature breeding age (see p. 156). • Nutritional status (body condition score) and growth rate. Although there are no data relating to the effects of nutritional status on the onset of puberty, this does occur in other species and there is no reason to suppose that it would not be similar in the horse. In ruminants, nutritional deprivation usually results in delayed onset of puberty. Whether precocious puberty occurs in response to high energy and rapid growth and weight gain in horses is uncertain. Most breeders are not driven to encourage early onset of puberty in mares in particular and prefer to rely on natural events.7 • Contact with other horses of breeding age (pheromone-governed). Typically, the effects of pheromones may be altered by the endocrine status of the animal and it may be that these factors have little overall influence on the onset of puberty itself. • A disparity between the breeding season arbitrarily imposed by racing and performance industries and the physiologic breeding season of mares. • Failure to select for fertility, including retention of breeding stock with heritable defects that reduce fertility (e.g. bad vulvar confirmation, cryptorchidism). • Prohibition of artificial insemination (in some breeds only and in particular the thoroughbred). During winter, most mares pass through a period of anestrus or sexual inactivity, when neither follicles nor corpora lutea are present. The response to the stallion is neutral (being neither receptive nor rejective). Day length is the primary factor controlling this seasonal ovarian activity.8,9 Long day length (15–16 hours), such as occurs during the summer, stimulates ovarian activity. The effects of day length are mediated negatively by melatonin secretion from the pineal gland within the brain. Prolonged periods of high melatonin secretion during the short winter days suppress gonadotropin-releasing hormone release from the hypothalamus. Increasing day length in spring causes shorter periods of high melatonin production, allowing an increase in the frequency and amplitude of pulsatile gonadotropin-releasing hormone release. Seasonal effects on ovarian cycles can be divided into three phases: 1. The ovulatory phase: the period from first ovulation in the spring until the last ovulation in the autumn. 2. Anestrus: the period of ovarian inactivity during the winter months. 3. Transition: the period of irregular or prolonged estrus receptivity that occurs in early spring or late autumn. In both northern and southern hemispheres, 75–80% of mares demonstrate seasonally polyestrous behavior, whereas 20–25% have estrous cycles all year. The percentage of mares that cycle throughout the year increases nearer the equator and year-round cyclicity is more common in Arabian mares.10 During anestrus, the endocrinological functions that govern cycles essentially are shut down. As a result of a shortened photoperiod, small and infrequent pulses of gonadotropin-releasing hormone from the hypothalamus lead to baseline levels of plasma luteinizing hormone concentrations. Baseline concentrations of plasma follicle-stimulating hormone remain relatively high but fluctuate randomly during anestrus, presumably as a result of the lack of any negative feedback effect from ovarian inhibin and estrogen.11 In most mares, the changeover from anestrus to a fully functional ovulatory phase is gradual. This transition is characterized by the re-establishment of endocrine function, erratic sexual behavior, and follicle development without accompanying ovulation. Increasing follicular activity causes the ovaries to enlarge considerably in comparison to their size during anestrus. There is a noticeable change in consistency as multiple small follicles begin to grow (Fig. 5.8). A springy resilience is evident on deep palpation, in contrast to the dense, firm consistency noted during the anovulatory phase. Fig. 5.8 Normal ovary (spring transition ovary). Note the numerous small atretic follicles with a few medium-sized follicles developing. In pony mares, three to four follicles greater than 30 mm develop sequentially during transition over a period of several weeks. The first and second follicles usually regress, whereas the third or fourth follicle will undergo the first ovulation of the season. Estrogen production is the hallmark of the follicles that successfully ovulate.12 Most of the time, the first follicle of the year that ovulates is accompanied by uterine edema visualized on ultrasonography (see p. 217). The increase in day length during spring leads to an increase in both the amplitude and the frequency of pulses of gonadotropin-releasing hormone from the hypothalamus.11 Plasma progesterone concentrations are less than 1 ng/ml until after ovulation, when they rise sharply. Plasma estrogen concentrations also remain low during anestrus but then rise coincidentally with the wave of follicular growth that precedes the onset of the first ovulation.13 The duration of the individual components and the length of the total estrous cycle vary greatly (Table 5.1). The greatest variability is at the beginning and end of the ovulatory phase. Therefore the duration of estrus is shortest during the peak of the ovulatory season and corresponds with the peak of fertility. Breeding mares at the optimal time early in the imposed breeding season require closer management than breeding mares at the peak of the physiologic breeding season. For example, it can be assumed that ovulation occurs 24 hours before the end of estrus and that sperm is viable for 48 hours within the mare’s reproductive tract. Without palpation for follicular development, if breeding commences on the second day of estrus, three breedings are necessary to adequately expose mares with an 8–9-day estrus. Mares with a 4-day estrus need only be bred once. Due to this variability, if palpation is not or cannot be used, mares are covered on the second day and then every other day until estrus has ended. This illustrates the value of accurate palpation in avoiding unnecessary stallion services. Three main groups of hormones are involved in control of the estrous cycle. The ‘brain hormones’ (melatonin and gonadotropin-releasing hormone) convert external stimuli into direct stimulation of the pituitary gland. The ‘pituitary hormones’ [the gonadotropins (follicle-stimulating hormone, luteinizing hormone), prolactin, and oxytocin] exert a direct trophic action on the ovaries, uterus, and other parts of the genital tract. The ‘genital or sex hormones’ [estrogen, progesterone, inhibin, and prostaglandin F2α (PGF2α)] are secreted in response to stimulation by pituitary hormones and control functional changes in the genital tract and behavioral changes in the animal. They feed back positively and negatively on hypothalamic and pituitary hormone secretion rates (Fig. 5.9).14 Fig. 5.9 Hormonal control of the estrous cycle in the mare. AP, anterior pituitary; LH, luteinizing hormone; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone. • Follicle-stimulating hormone stimulates the initial growth of follicles in the ovaries during diestrus. • Luteinizing hormone stimulates maturation of the follicles, maturation of the oocytes within the follicles, and ovulation during estrus. During the physiologic breeding season of mares, follicle-stimulating hormone concentrations peak twice during each cycle at about 10–11-day intervals (Fig. 5.10). Follicle-stimulating hormone secretion is stimulated by increased day length and suppressed by inhibin secreted by developing follicles. The first peak of follicle-stimulating hormone concentrations occurs near the end of estrus. This peak coincides with the luteinizing hormone peak at or soon after ovulation. The second follicle-stimulating hormone surge is seen in mid-diestrus, when follicular activity is at its lowest. It is theorized that this surge is responsible for initiating the wave of follicular growth that provides the ovulatory follicle during the ensuing estrus. Follicles stimulated after the first surge of follicle-stimulating hormone reach the luteinizing-hormone-dependent stage between days 5 and 7 of the estrous cycle and become atretic if luteinizing hormone is not available.16 Plasma concentrations of luteinizing hormone are low between days 6 and 15 (Fig. 5.10) after ovulation because of the negative feedback action of progesterone on the hypothalamus, causing the suppression of gonadotropin-releasing hormone. Blood concentrations start to rise near the beginning of estrus (day 17) as the suppressive effects of progesterone are removed and probably as a consequence of the positive stimulatory action of estrogen on gonadotropin-releasing hormone pulse frequency. Concentrations of luteinizing hormone peak about 2 days after ovulation and then decline slowly. Because of its prolonged half-life, luteinizing hormone reaches baseline concentrations at about day 5 or day 6 after ovulation.3 • Softening and relaxation of the cervix. • Increased fluidity and volume of uterine and vaginal secretions. It also acts on the brain, stimulating behavioral changes of estrus and having both positive and negative feedback on gonadotropin-releasing hormone (see Fig. 5.9). Shortly before the luteinizing hormone surge, plasma and urinary estrogens rise, reaching a peak 24–36 hours before ovulation. Estrogens are secreted mainly as estrone and 17β-estradiol. In the absence of concurrent high progesterone concentrations, high concentrations of estrogen cause estrous behavior. Progesterone is secreted by the corpus luteum during days 1–17 after ovulation. Progesterone causes contraction of the cervix and increased viscosity of vaginal secretions. It stimulates proliferative changes in the uterine luminal epithelium and endometrial glands and acts on the brain to induce the rejection-type behavioral response shown by mares in diestrus. Plasma progesterone concentrations are low (less than 1 ng/ml) throughout estrus but rise sharply after ovulation, reaching values of 1.5–2.5 ng/ml by 24 hours and 2–5 ng/ml by 48 hours post ovulation (day 0). Peak concentrations of 8–20 ng/ml are attained by days 5–8 after ovulation, and these values remain high until luteolysis commences at days 14–15 after ovulation (Fig. 5.10). The mid-cycle release of follicle-stimulating hormone (Fig. 5.11) stimulates follicular development. Around days 16–18 after ovulation, near the beginning of estrus, a few follicles (greater than 25 mm in diameter) are present, and one follicle is associated with the luteinizing hormone surge beginning on day 16 or 17 after ovulation, when levels of follicle-stimulating hormone are lowest. Resulting estrogen production from these growing follicles initiates the physical and behavioral changes of early estrus. As the dominant follicle approaches maturation, a large preovulatory surge of estrogens produces a positive feedback on the pituitary, continuing the rise in luteinizing hormone concentrations. In contrast to females of other species, mares have a prolonged surge in luteinizing hormone levels that often lasts for 7–8 days and usually peaks after ovulation (Fig. 5.11). Because it is common for multiple follicles to develop during estrus, this long exposure to luteinizing hormone may account for the large number of multiple ovulations that occurs. In its embryonic development, the cortex of the equine ovary folds at its hilus and becomes surrounded by medullary tissue. Mechanically, this only allows ovulation through the hilar area or ovulation fossa. Follicles are palpated on the general ovarian surface, but the follicle collapses at ovulation and forces the ovum through its tract to the ovulation fossa. Follicles usually increase rapidly in size (about 5 mm in diameter per day) before ovulation. Mares weighing 400–550 kg often ovulate from follicles 45–65 mm in diameter, whereas smaller mares weighing 225–350 kg may ovulate from follicles 35–45 mm in diameter.17 Ovulation usually occurs during evening or night hours, with approximately 75% of mares ovulating between 4:00 p.m. and 8:00 a.m. The incidence of multiple ovulation reportedly varies from 14.5% to 42.8%.18 Endocrine changes after ovulation are quite rapid in most mares, and physical and behavioral changes follow very shortly (Table 5.2; see also Fig. 5.7). Some luteinization probably occurs before ovulation; however, estrogen concentrations drop drastically when the follicle collapses and the corpus luteum develops quickly. The resulting reversal in circulating estrogen:progesterone ratio causes the rapid cessation of estrous behavior. At this point, progesterone rather than estrogen dominates behavior and the physical character of the tubular genitalia. The corpus hemorrhagicum is palpable per rectum during the first 2–3 days after ovulation as a soft, spongy structure on the surface of the ovary. As it matures into a corpus luteum and the blood within the corpus hemorrhagicum clots, the consistency changes to that of a rubbery, firm structure that is difficult to distinguish from a firm follicle (Fig. 5.12). The ultrasonographic appearance of the corpus luteum is characteristic and is easily identified throughout its lifespan. Fig. 5.12 Normal ovary, showing mature corpus luteum (brownish/yellow tissue; arrowed), smaller corpus luteum and atretic follicles. Note the thickened walls of atretic follicles. Detailed breeding records may be very helpful in predicting when the mare is likely to come into estrus, although there may be variations in cycle length during the season and variations may also arise from early embryo loss, etc. (see p. 163). By far the most efficient method for estrus detection is to tease each mare individually with a stallion, but mares can also be teased by teasers, or on occasion by geldings (see p. 153). The interrelationship between a mare and stallion during estrus is probably more dependent on smell and taste rather than on sight. However, teasing is usually restricted to one or two 5–10-minute periods each day and this is clearly an artificial circumstance. While most mares will either show signs of estrus or diestrus (rejection behavior and absence of clinical evidence of estrus), some mares may not respond normally to this narrow time scale and often appear to be in diestrus throughout the cycle. The extent of estrus ‘showing’ is variable. Some respond quickly to contact with the stallion (often even on initial approach), but other mares may take considerable time to settle to the behavior pattern. • Maiden mares may also be difficult, especially those that are nervous and excitable; they may be reluctant to show estrus and may then not respond well even when teased individually. • Mares do not show any overt sexual behavior (e.g. mounting each other) when grouped with other mares and overt changes in the physical characteristics (mucous secretions) of the vulva are not usually obvious. • Mares with a high androgen concentration (resulting from administration of male hormones or anabolic steroids or certain ovarian tumors) may show masculine behavior rather than estrus.19 • Estrus signs may be much stronger in the presence of an active stallion. Estrus in paddock or free-bred mares will be detected quickly by the attending stallion(s). • Mares with foals at foot may not show strong signs of estrus, either if the foal is separated from her at the time of teasing or if the foal is physically beside the mare during teasing. This is caused either by separation anxiety or by protectiveness, respectively. Estrous behavior is defined as the pattern that results in acceptance of the approach of, and mating by, a stallion. Usually a mare is considered to have been in estrus if she shows 4–6 days of estrous behavior followed by a period of 10–14 days of diestrus during which she should show no receptivity to the stallion and no overt clinical signs of estrus20 (see below). Individual mares may habitually show strong or weak estrous signs. In the latter cases, supportive testing (see alternative methods below), dates and records can be helpful. Behavioral changes typical of estrus include: • Docility (although behavior can be unpredictable). • Ears relaxed either forward or in neutral position. • Vocalization in the form of squealing often occurs with laid back ears when the mare is first approached by the stallion. • Tail raising and swishing with adoption of a stance similar to urination but maintained for longer periods and without evidence of straining. The tail is usually held slightly to one side. • Lengthening and eversion of the vulvar lips with clitoral eversion (‘winking’). • Squatting and frequent squirts of urine (usually with a characteristic odor and yellowish cloudy appearance). Vaginoscopic examination (Fig. 5.13) can be used to detect estrus (and diestrus) with some accuracy but it is a semi-invasive method and can cause problems with vaginal inflammation. The normal state of the cervix changes during estrus (and during pregnancy).21 During estrus the cervix shows hyperemia (a red or dark pink color). The mucosa is edematous (moist-looking and thickened) and the cervix itself is relaxed and lies on the floor of the vagina. The mucus consistency correlates closely with the rise in blood estrogen and is recognized as an increased shininess of the mucosal surfaces. Introduction of the speculum is relatively easy due to increased mucous secretion during estrus but commonly causes a significant increase in blood vessel diameter and increased redness of the mucosal surfaces. A classification system has been proposed for the description of the cervix during examination.22 This has advantages because any repeat examinations can be considered with the previous ones. This is just one classification scheme; many others have been created and can be used to describe the differences in cervical tone during estrus and diestrus. As long as the examiner uses the same classification scheme consistently, it will be useful for comparison during repeated examination of the reproductive tract. The value of an effective and thorough ultrasonographic examination cannot be overstated. With the use of a high-quality scanner the reproductive tract can be examined with sufficient detail to provide important information. The uterus of mares in estrus will have a ‘cartwheel’ appearance as visualized on ultrasonography (see Fig. 6.8), due to the increase in edema in the uterine wall. The ‘cartwheel’ breaks up approximately 24 hours prior to ovulation and can be used to identify mares in estrus and to time breeding. Mares in diestrus will have a homogeneous appearance to the uterus and a corpus luteum on one of their ovaries. Pathology, such as intrauterine fluid or lymphatic cysts, that can not be identified by rectal palpation can easily be visualized by ultrasonography. Breeding soundness examinations are performed to determine a mare’s suitability as a broodmare and for identifying causes of infertility. Portions of the examination are performed routinely on normal mares during the breeding season to determine when they should be bred. Common use of new technologies, such as artificial insemination of mares with shipped, cooled semen or frozen semen and embryo transfer, require that the veterinarian excel in reproductive examination skills. A complete breeding soundness examination (see Fig. 5.14) is most commonly performed to identify a cause of infertility. Although in purely dictionary terms infertility can be defined as the failure to produce an offspring (whether or not that reflects failure to conceive), the stud farm definition is usually taken one stage further: that is, failure to produce a productive offspring that achieves its desired use and has the ability (if not the opportunity) to breed itself once it has reached breeding age. 1. Obtain a full reproductive and other history of the mare. • This should include as much detail as is practicable and should not be limited to the reproductive history. • There are many aspects of reproduction that are influenced by diseases and accidents/injuries involving other body systems. • Medications of all types can be responsible for difficulties with conception and maintenance of pregnancy. • A full history of the fertility rates and changes in these for the whole stud should be taken. 2. Perform a complete physical examination. • The examination of the infertile or problem mare should not be limited to the reproductive organs as there are many secondary reproductive effects from other diseases (e.g. equine Cushing’s disease/pituitary adenoma, endotoxemia). 3. Perform a full reproductive examination in a logical order. • Inspect the external genitalia. • Rectal examination (see p. 133). • Ultrasound examination of the reproductive tract. • Collect clitoral swabs (clitoral sinus and fossa). • Speculum examination of vagina and collect cervical and endometrial swabs for culture and cytology (make smears immediately after collection). • Manual examination of vagina, vestibule and cervix. • Endometrial biopsy (see p. 144). 4. Obtain peripheral blood samples for hormone analysis/chromosomal analysis.
THE MARE
REPRODUCTIVE ANATOMY OF THE FEMALE GENITAL TRACT
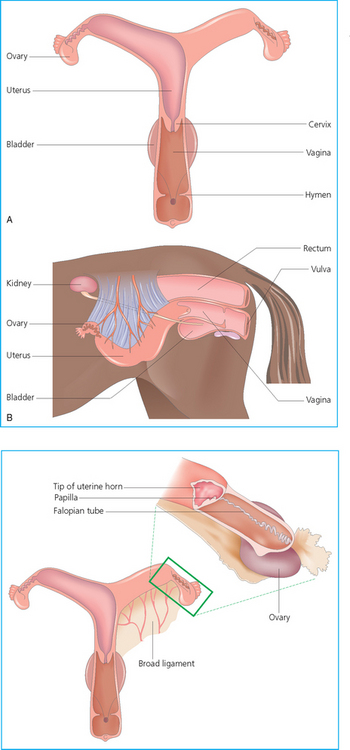
Vulva and vestibule
Clitoris
Cervix
Uterus and broad ligaments
The ovaries
Oviduct
PHYSIOLOGY
PUBERTY
THE NORMAL ESTROUS CYCLE AND SEASONALITY
Anestrus
Transition
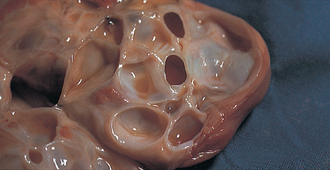
The ovulatory phase
CYCLIC EVENTS OF THE OVULATORY PHASE
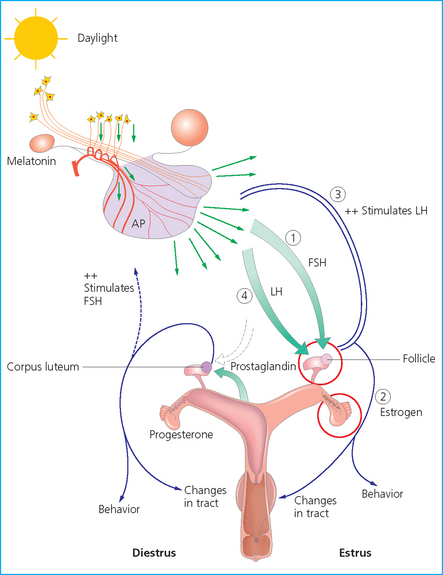
Pituitary function
Follicle-stimulating hormone
Luteinizing hormone
Ovarian function
Early estrus
Late estrus
Ovulation
Diestrus
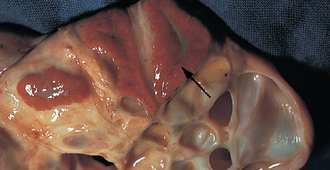
RECOGNITION OF ESTRUS IN THE MARE
Alternative methods of estrus detection
Vaginal examination
Rectal and ultrasonographic examination
EXAMINATION OF THE FEMALE GENITAL TRACT
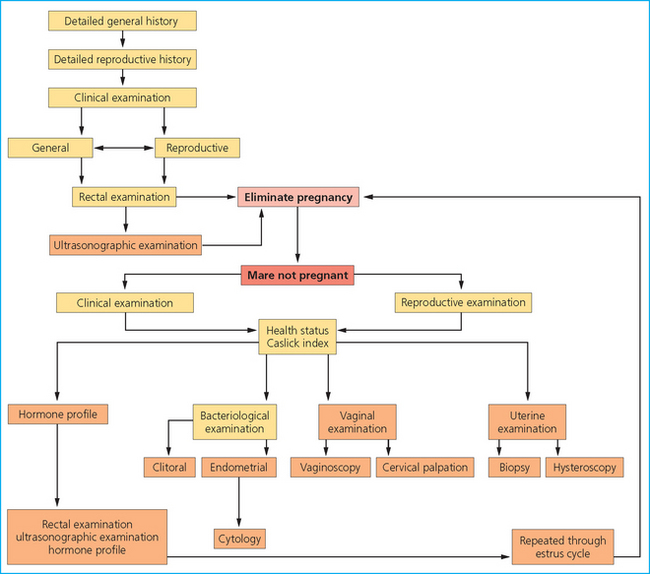
BREEDING SOUNDNESS EXAMINATION
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


THE MARE