Fig. 9.1
Schematic drawing of female koala reproductive tract; (A) Ventral aspect and (B) Lateral aspect. Bl—bladder; Bu—bursa; Cx—Cervix; Fi—Fimbria; In—Infundibulum; Lv—Lateral vagina; Ms—Medial septum; Od—Oviduct; Ug—Urogenital strand; Ur—Ureter; Us—Urogenital sinus; Vc—Vaginal cul-de-sac (Modified from Obendorf 1988)
The reproductive cycle of the koala is reasonably well understood (Johnston et al. 2000b, c, 2004); endocrinology of the pregnant and non-pregnant koala cycle is illustrated in Fig. 9.2. While ovulation appears to be induced by coitus, the exact mechanism of induction is still unresolved as there is some evidence that semen may possess some type of ovulation-inducing biochemical, similar to what has been described in the camel (Johnston et al. 2004). Like the felidae, there are three possible types of koala oestrous cycle. The first involves the follicular phase without induction of ovulation and a luteal phase and is referred to as interoestrus (Fig. 9.2a; Johnston et al. 2000b). The female displays oestrus for approximately 10 days and if mating does not occur, oestradiol secretion subsides and the female takes approximately 33 days to come back into oestrus again. If the female mates with a male, an LH surge occurs approximately 28 h after mating and plasma progesterone levels commence to rise and peak about 28d after mating (Fig. 9.2b). If pregnancy is not successful, the female will come back into oestrus approximately 50 days from the previous oestrus (Fig. 9.2b). If a pregnancy does result, gestation will occur over a period of 35 days (Fig. 9.2b) and following parturition, the female will fail to return to oestrus, most probably because of the suckling stimulus of the pouch young preventing further ovarian activity. This detailed understanding of the physiology of the koala oestrous cycle and ovulatory pattern has been fundamental to the success of the artificial insemination program, in terms of the most appropriate timing for insemination, but also in developing procedures to induce ovulation. We are currently in the process of sequencing Koala GnRH, FSH and LH for the purposes of developing specific antisera so that these protein hormones can be monitored throughout the cycle, pregnancy and early lactation; we are also sequencing the respective receptors of these hormones. This information will also help us design or select the most appropriate GnRH antagonists for work oestrous synchronisation.
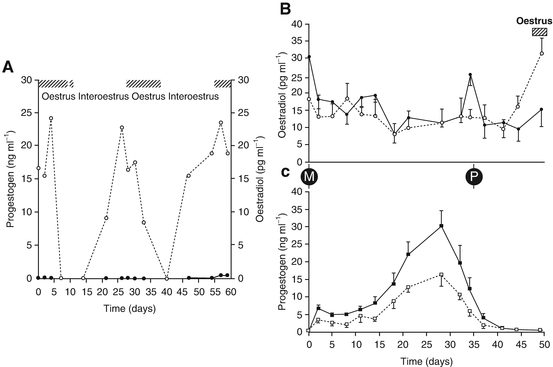

Fig. 9.2
Reproductive endocrinology of the koala. (A) Oestradiol (open circles) and progestogen (closed circles) secretion during an anovulatory oestrous cycle; (B) Oestradiol secretion post-mating during pregnancy (closed circle) and a non-sterile oestrous cycle (including luteal phase; open circle); (C) Progestogen secretion post-mating during pregnancy and a non-sterile oestrous cycle (including luteal phase). Cross-hatching—behavioural oestrus; M—mating; P—parturition (From Johnston et al. 2000b)
Oestrus in the captive koala is one of the most visually and auditory overt behaviours reported for any marsupial (Johnston et al. 2000a; Feige et al. 2006). In a procedure known as “teasing”, an adult male is brought into an all female enclosure. The male is initially presented to each female by the zookeeper by holding the male at eye-level with each female so that all females are aware of the male’s presence. Those females that are not in oestrus will typically reject the male with an aggressive vocalisation and or by physically striking out at the male with their forelimb. Females in oestrus will show interest in the male and some will respond by immediately initiating oestrus-related behaviours. The male is then placed on the enclosure floor where he undergoes prescriptive pre-copulatory behaviours including urination, scent marking a tree pole within the enclosure with his scent gland, and most characteristically, vocalising with deep guttural bellows. The bellow of the male is typically the trigger that initiates the expression of oestrus in the female and on hearing the bellow the oestrus female’s excitement level rapidly increases. Interestingly, the female will not necessarily approach the bellowing male but more typically, locates other females in the enclosure with whom she attempts to mount and copulate. The oestrous female will normally take on the male role during pseudo-copulation, establishing a neck bite to the nap of the neck to stabilise the female that is being mounted and orientating herself as if she were the male performing the copulatory act (Feige et al. 2006; Fig. 9.3). The mounting female will display the full range of male copulatory behaviours, including pelvic thrusting, a period of stillness, which is similar to the period when the male would be ejaculating, and a finally, a neck bite to the shoulder as a signal of disengagement; some females will even bellow at the end of the pseudo-mating attempt. Remarkably, the female that is being mounted will take on the normal female role during this process and demonstrate lordosis (arching of the backbone and backward tilt of the head), a period of stillness while the female is mimicking male thrusting behaviour and then a period where she shows convulsive jerking of her body associated what is presumably a mimic of ejaculatory behaviour (Feige et al. 2006). Typically, the oestrous female seeks out other females that are either in oestrus or coming into or out of oestrus, as females that have been mounted will then proceed to serve the female who has just finished mounting. Given that oestrus in the koala is on average 10 days of a 33 day cycle, it is not all that surprising that their will be more than one or two oestrous females in the enclosure at the same time. Other behaviours that are associated with koala oestrus include, bellowing behaviour by the female, convulsive jerking of the body in what has been likened to uncontrollable “hiccoughing”, urination and increased agitation or restlessness (Johnston et al. 2000b). Koalas are normally very sedentary in captivity but there is a notable change in movements and activity during oestrus. As in a range of domestic species, koalas also appear to demonstrate a standing oestrus in which the female will stand still (receptivity) while the male ensues a copulatory position. In some cases, the female may even back down “rump first” in the face of the male on the same pole in what appears to be a form of “cloacal presentation”. All of these behaviours are unmistakable indicators of oestrus for the zookeeper, who along with help from the teaser male koala can determine the daily reproductive status of the female from behavioural observations. Some zookeepers have even learnt to mimic the bellow of the male koala or play recordings of the male bellow to the female and this has been used for effective oestrus detection. All these “oestrus” behaviours have been linked to an elevated secretion of oestradiol (Johnston et al. 2000b). The ecological significance of captive koala homosexual behaviour is difficult to comprehend but is probably an artefact of captivity, as the behaviour has never been observed in the wild. Feige et al. (2006) have suggested that it may be associated with sexual excitement linked to high levels of oestradiol in the systemic circulation and that this may be the physiological mechanism that causes wild koalas to seek out males outside of their normal home range.


Fig. 9.3
Pseudomale copulatory behavior in three oestrous koalas. Note the homosexual behaviour of the middle and lower females (From Johnston 1999)
7 The Reproductive Biology of the Male Koala
The reproductive anatomy of the koala has been well described by Temple-Smith and Taggart (1990) so that only a brief overview will be presented here. As for most marsupials, the male Koala has a pre-penile scrotum; the scrotal; skin being non-pigmented and covered with short hairs. The importance of the scrotum for thermoregulation of the testes has not yet been investigated. The flaccid penis is maintained within the prepuce in an s-shaped configuration. The glans penis is covered proximally with prominent keratinised spines but the precise role of these spines has yet to be clearly determined—we have speculated that they are likely to have role in stimulating reflex ovulation along with ovulation factors in the semen (Johnston et al. 2004). In the centre of the chest of the male is a sternal gland which produces an oily sebaceous and odorous secretion; the scent gland appears to be androgen dependent and its activity increases leading up to and during the breeding season (Allen et al. 2010).
The testis of the koala is supplied by a rete mirabile of over 100 blood vessels and five large lymphatics. The testis is ellipsoidal and small in comparison to that of other marsupials, weighing only approximately 0.07 % of total body weight (Johnston 1994); the significance of small testicular size on the reproductive strategy and behavioural ecology of the koala requires further investigation. Oishi et al. (2013) has recently described the quantitative testicular histology and the dynamics of the seminiferous cycle in the koala and wombat in which we report both species possessing eight stages of cellular associations. Interestingly, the koala (≈33 %) differs from the Southern Hairy-nosed Wombat in typically having a greater proportion of interstitial testicular parenchyma. Temple-Smith and Taggart (1990) refer to the koala as possessing a Type 3 pattern of organisation with large tracts of Leydig cells completely isolating adjacent seminiferous tubules. The Sertoli cells of the koala contain nuclei that are 5X larger than that those in eutherian species and which possess unusual crystalloid inclusions, the precise function of which is still unknown. Testicular volume of koalas in SE Queensland changes throughout the year in both wild and captive populations with an increase over spring and summer and a decrease in autumn and winter (Allen et al. 2010).
Spermiogenesis in the koala has a number of unusual features including; the formation of a proacrosomal granule within the acrosomal vacuole, an uneven condensation of chromatin and a unique flattening of the sperm nucleus which results in the wide variation observed in sperm head morphology (Temple-Smith and Taggart 1990). The acrosome is located in a dorsal nuclear cleft and there is an unusual ventral neck insertion of the mid-piece into the sperm head (Harding et al. 1979; 1987; Harding and Aplin 1990). Harding and Aplin (1990) have used these unusual features of the spermatozoon to review the phylogenetic position of the koala (Harding et al. 1987; Harding and Aplin 1990) confirming a close relationship with the wombat.
The gross anatomy of the Koala epididymis and associated vasculature is similar to that described for other diprotodontid marsupials (Temple-Smith and Taggart 1990). The caput epididymidis is expanded in shape and connected via a corpus segment to a bulbous cauda epididymidis. The epithelium of Koala epididymis consists of four basic cell types; principal, basal, mitochondrial rich and electron-translucent, but they have no specialised regions of phagocytic principal cells. As for most mammals, Koala sperm appear to gain the capacity to become motile as they move into the cauda epididymidis. Fluid absorption in the cauda epididymidis results in a higher sperm concentration than that found in the caput epididymidis (Temple-Smith and Taggart 1990). While there is a marked increase in the curvature of the sperm head and a folding of the acrosomal surface and condensation of the accessory cytoplasmic droplet to within the hook of the sperm head (Hughes 1977), there appear to be no other morphological changes to the Koala spermatozoa during epididymidal transit; this is in sharp contrast to changes in sperm morphology that occur during epididymidal transit in other marsupials (Harding et al. 1983; Rodger and Mate 1993).
The ductus deferentia run from the neck of the scrotum through the inguinal canal and into the abdominal cavity, emptying finally into the cranial portion of the prostatic urethra. The ductus deferens is non-specialised, non-glandular, with no seminal vesicles or ampullae (sperm storage organs in eutherian mammals). The Koala prostate is pyriform and can be divided into three histologically distinct regions, the functional significance of which have yet to determined. A short membranous urethra connects the prostate to the penile urethra. At the distal extremity of the membranous urethra and in close association with the penile crura and bulbs are three pairs of bulbourethral glands. Glands BII and BIII are filled with a thick, clear, mucin-like secretion that is strongly eosinophilic (Temple-Smith and Taggart 1990). Although Mitchell (1990) noted the presence of paracloacal glands in koalas, he gave no anatomical details or references. It is likely that the prostate provides the bulk of seminal plasma in koala semen, while the bulbo-urethral gland secretions have an important role in producing a seminal plug. In SE Queensland, a study of post-mortem specimens entering koala hospitals revealed no seasonal change in the size of the prostate but an increase in bulbo-urethral gland volume over spring, a decrease over summer and autumn and an increase towards the end of winter (Allen et al. 2010).
Like most mammals, plasma testosterone secretion in the Koala is highly episodic (Johnston 1999). The highest concentration of testosterone appears to occur in the koala during periods of male dispersal just prior to the breeding season, and not as may have been expected, during the breeding season (McFarlane 1990; Handasyde et al. 1990; Allen et al. 2010). A rise in testosterone concentration is coincident with the onset of bellowing, prior to the commencement of breeding activity (Handasyde et al. 1990). In order to obtain a more reliable measure of the testosterone secretory capacity of the koala testis, a GnRH or hCG stimulation test can be employed (Allen et al. 2006). This approach was used to demonstrate a seasonal change in testosterone secretion of both captive and wild koalas in southeast Queensland with a peak in spring and nadir in autumn (Allen et al. 2010).
8 Semen Collection
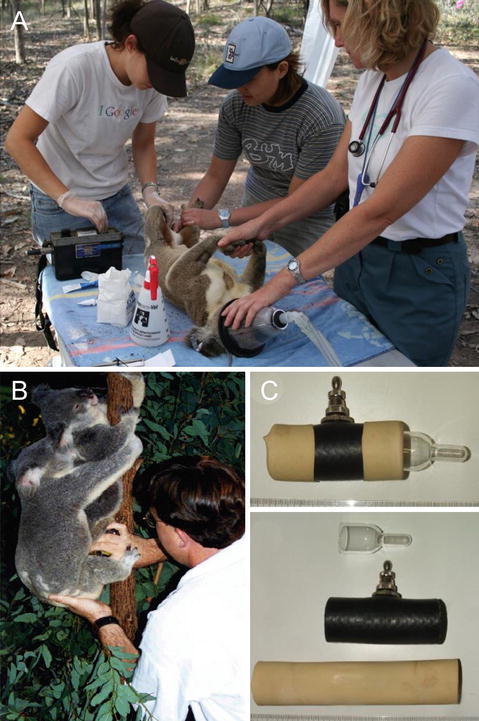
Our first major step towards the development of a successful AI program in the koala was the ability to reliably collect semen in sufficient volumes to be used for insemination. Semen collection in the koala using electro-ejaculation was first described by Wildt et al. (1991) and later by Johnston et al. (1994); the procedure has been extremely effective with greater than 90 % of semen collection attempts resulting in spermatozoa. The procedure is field applicable (Fig. 9.4a) and a recent study by Allen et al. (2010) showed that it was possible to repeatedly collect semen from both captive (monthly) and wild koalas (every 6 weeks) from the same individuals. However, there are also disadvantages with respect to the use of the procedure that need to be acknowledged, including the requirement for the koala to be anaesthetised (which can be problematic), production of semen with variable compositions of seminal plasma, lower sperm concentration, urine contamination and in rare cases rectal trauma through improper placement of or overstimulation from the rectal probe. We have used the electro-ejaculation procedure to determine semen quality and provide semen for studies of sperm physiology, preservation and artificial insemination.
A major break through in determining the appropriate parameters of koala semen for AI was the ability to collect semen using an artificial vagina (Johnston et al. 1997; Fig. 9.4b, c). Before attempting the procedure we carefully observed the natural mating behaviour of the koala to inform us of how we might engage the animal to serve the artificial vagina, for a major problem that we needed to overcome, was the fact that the male koala thrusts his penis into the female urogenital sinus in a vertical direction and that semen was presumably ejaculated in a similar trajectory against “gravity”. Contributing to the success of the technique was the tractability of both male and female koala to tolerate human presence during mating activity. In fact, it was not uncommon for zookeepers at Lone Pine Koala Sanctuary to assist the male to direct his penis into the urogenital sinus of the female during natural mating. Prior to attempting semen collection with the AV, zookeepers had reported to us that during movement of koalas from one enclosure to another that males held against the abdomen of the zookeeper would often ejaculate during transit. Captive koalas are handled from an early age and there is no doubt this early exposure to the zookeeper resulted in animals with a high tolerance to intervention and manipulation. We had observed that during copulation that the male was very focussed on securing the female in an appropriate position by establishing a neck bite and using his forearms but that his hind limbs were rarely used to support his hold. Based on our observations of natural mating behaviour, we constructed a koala AV from a ram artificial vagina that was adjusted to the length of the koala penis. After preparing the AV with hot water (42–45 °C) and inflating it with air, the female is placed on a single tree-pole so that she was secure for mating at approximately eye height of the collector. The male koala is then brought into the female’s enclosure, placed on the floor and allowed to conduct his normal pre-copulatory behaviour, including sternal gland scent marking, urinating and bellowing, all of which stimulates oestrus behaviour in the female. The male is then placed on the tree pole directly below an oestrus female and allowed to establish his mating position. The male typically manipulates the female into position while at the same time establishes penile erection. Once the penis is fully erect the collector would step in and direct the male’s penis into the warm AV; the male in most cases appeared to be unaware that he was serving the AV rather than the female and the female continues to display her normal copulatory behaviour. The male then thrusts vigorously into the AV and completes this behaviour with two strong final ejaculatory thrusts, which appear to signal the commencement of ejaculation. At this point, the collector lifts the rump of the male slightly horizontally so as to direct the flow of semen into the collection vial; this manipulation was possible because the hind limb of the male is not used for support during copulation. Once ejaculation is completed the male disengages the female by biting her shoulder or arm and which results in an aggressive response by the female and separation. During disengagement the collector removes the AV from the male’s penis. Semen collected by the AV provides a means of determining a more natural estimate of seminal characteristics, including semen volume (≈1 mL), pH and sperm concentration; all these seminal parameters are crucial for developing appropriate methods of artificial insemination. While this method of semen collection (58 %) is less reliable than electro-ejaculation (96 %) it does offer the additional advantage of observing structure physical defects in breeding soundness and assessment of male sexual drive. To date the koala remains the only marsupial for which the AV has been used successfully.
It is also possible to recover spermatozoa from post-mortem specimens collected from koala hospitals and veterinary clinics and use these gametes in assisted breeding programs (Johnston and Holt 2001; Johnston et al. 2013b). Each year in SE Queensland, 100 s of koalas are euthanased because of disease or trauma; this is such an appalling waste of genetic material that could potentially be utilised for genetic exchange programs. While this procedure has been described and proven in the Common Wombat (MacCallum and Johnston 2005) and Southern Hairy-nosed Wombat (Johnston unpublished observations) there are currently no published studies of gamete recovery in the koala. Although the yield of recovered epididymidal spermatozoa is likely to be less than that of the wombat, we nevertheless, have preliminary data that indicates that this approach is feasible. Dief (2011) has recently noted that while ascending chlamydia infection can cause orchitis and epididymitis in the koala, some animals have prostatic infection only. These animals can be used for sperm collection and used directly for AI or for cryopreservation. We are also exploring the use of semen “clean-up” technology to remove or destroy viable chlamydial elementary bodies.
9 Semen Evaluation
The koala ejaculate collected by electro-ejaculation offers up some challenges for seminal analysis as the sample is typically very viscous and contains a high proportion of prostatic and bulbo-urethral secretions; on rare occasions the semen may even coagulate preventing further manipulation. In order to assess semen quality, the raw semen is typically diluted 1:10 in a Tris-citrate glucose or fructose diluent at room temperature. The raw semen sample has little mass activity, the pH is typically neutral and osmolality is in the order of 300–350 mOsm kg−1. Sperm concentration of koala semen collected by AV was typically higher (165 × 106/ml) than that collected by electro-ejaculation (83 × 106/ml).
The assessment of sperm motility is conducted on a diluted sample on a warm stage set to 35 °C (approximate body temperature of the koala) and the percentage of sperm swimming in a progressively forward manner is determined along with an assessment of the rate of sperm movement (0—no movement to 5—extremely rapid movement). In early studies and without the use of fluorescent microscope, viability (intactness of the plasma membrane) was assessed using nigrosin-eosin (Johnston et al. 1994; 1997) but this was later substituted for the use of SYBR-14 (live) and propidium (dead) stains; we found the use of nigrosin-eosin somewhat problematic when using diluents containing egg yolk. More recently we have turned to the use of the JC-1 stain (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbezimidazolyl carbocyanine iodide) for the assessment of mitochondrial membrane potential, which provides additional insight to quality of sperm motility. Sperm motility of semen collected by electro-ejaculation or by artificial vagina is usually in the order of 70–80 % progressive motility. The live-dead and JC-1 stains can be used in combination to provide simultaneous assessment of both parameters (Fig. 9.5A). Interestingly, sperm motility of wild and captive koalas was highest in winter, as was the post-thaw survival of cryopreserved spermatozoa from the same animals (Allen et al. 2010).
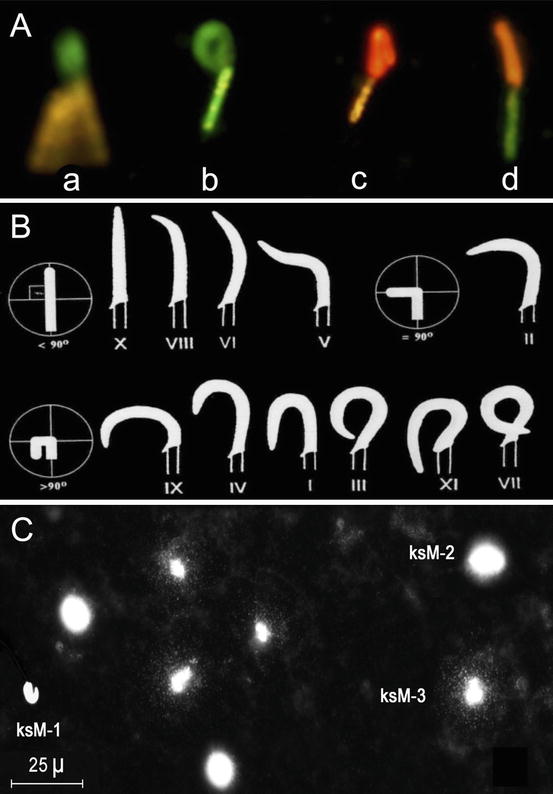

Fig. 9.5
Evaluation of Koala spermatozoa; A. Assessment of live (Sybr14—green) and dead (Propidium Iodide – red) sperm cells in combination with the assessment of mitochondrial membrane potential using the JC-1 stain (orange—high MMP; green—low MMP) (From Zee et al. 2007); B. Koala sperm nuclear morphotypes (I–XI); C. Assessment of koala sperm DNA fragmentation – KSM-1, rod-shaped nuclei without a halo of chromatin dispersion; KSM-2, round and rod-shaped nuclei showing a compact halo of chromatin dispersion about a nuclear core; KSM-3, sperm nuclei with enlarged halo and stellar chromatin corresponding to DNA fragments diffusing from the central core. (From Johnston et al. 2007)
One of most striking feature of the Koala spermatozoon is the extent of pleiomorphy in the head morphotype. Temple-Smith and Taggart (1990) identified two extreme and four common intermediate head morphologies from both testicular and epididymidal sperm, including a structural abnormality of the neck-midpiece region. Wildt et al. (1991) have identified ten nuclear morphotypes, while Johnston et al. (1994) identified 11 (Fig. 9.5B); mid-piece and principal piece structural abnormalities have also been described (Johnston et al. 1994); as to what specifically represents normal sperm head morphology in the koala has yet to be defined as is the exact mechanism of fertilisation given the unusual location of the acrosome within the curvature of the sperm head and thickened zona pellucida (Johnston 1999).
Recently we developed an assay for the assessment of sperm DNA fragmentation in the koala (Johnston et al. 2007; Zee et al. 2009a; Fig. 9.5C). This has been a first for a marsupial and was initially developed to explore reasons for post-thaw decondensation of koala sperm DNA. The assay has been appropriately validated with in situ nick translation and comet assay and is based on sperm chromatin dispersion in a microgel. Koala spermatozoa are loaded into a microgel on a microscope slide and treated with protein lysing agent to expose loops of DNA. Single stranded and double stranded DNA is then allowed to disperse in the microgel and DNA identified by fluorescence microscopy. Our data thus far suggests that DNA fragmentation occurs at a relatively low incidence (6.7 %; Johnston et al. 2013a) in captive population of koalas and that the DNA molecule is able tolerate prolonged periods of chilled storage. While our analysis revealed individual koalas with high levels of DNA fragmentation (up to 15.3 %) the significance of this phenomenon on fertility has yet to be determined. Given the relationship between chlamydia infection and high levels of DNA fragmentation in human spermatozoa (Gallegos et al. 2008), future studies are planned to investigate this relationship in the koala.
10 Semen Manipulation and Liquid Preservation
The practicality of an assisted breeding program based on artificial insemination is dependent on the ability to prepare and extend semen for insemination and the period of time that semen can be successfully preserved. Raw spermatozoa typically die rapidly ex vivo so that the semen must be rapidly diluted in a medium that is compatible for its evaluation and survival; this requires an analysis of the how spermatozoa tolerate physicochemical conditions such as pH, temperature and osmolality. Early in the development of our artificial insemination program we showed that koala sperm prefer a pH of 7–8, an osmolality of approximately 300 mOsm kg−1 and can tolerate a temperature range without a loss of sperm motility from 15 to 35 °C (Johnston et al. 2000a).
Contrary to evidence in most other marsupials, there is a small but significant decrease in sperm motility after rapid cooling of diluted semen from 35 to 5 °C, but compared to most eutherian species, koala spermatozoa would still be regarded as cold-shock tolerant and can be readily stored and manipulated at room temperature. In an attempt to explain the cold tolerance of marsupial spermatozoa we investigated the sperm membrane fatty acid composition in the koala and compared this to the common wombat and the eastern grey kangaroo (Miller et al. 2004). We discovered that koala sperm membranes had a high ratio of unsaturated/saturated membrane fatty acids compared to wombats and that sterol levels in marsupial sperm generally were very low. These marsupials were first to be examined for lipid membrane composition and highlight the dearth of information that exists for this taxon. We also employed a cryomicroscope to examine the effect of chilling on the sperm membrane directly (Zee et al. 2007). Using this technique we were able to view the effect of cooling to 5 °C on the plasma membrane and mitochondrial membrane potential simultaneously but there was no significant effect on the proportion of spermatozoa with high MMP or intact plasma membranes. Given the tolerance of koala sperm to cope with chilled temperatures, we are somewhat equivocal regards the effectiveness of egg yolk in the protection of the plasma membrane.
Tolerance of spermatozoa to changes in osmotic pressure can provide a guide to the sperm’s ability to cope with osmotic flux during cryopreservation. We originally determined that koala spermatozoa had a relatively narrow osmotic tolerance and were particularly susceptible to hypo-osmotic media < than 250 mOsm kg−1. Later we compared koala spermatozoa to that of wombat spermatozoa and found that koala spermatozoa were more susceptible to hyperosmotic damage than wombat spermatozoa (Johnston et al. 2006) both in terms of sperm motility and plasma membrane integrity. More recently, we have also examined the effect of osmolality on mitochondrial membrane potential, relaxed (swollen) chromatin and DNA fragmentation (Johnston et al. 2012). Plasma membrane integrity, chromatin relaxation and SDF appeared particularly susceptible to extreme hypotonic environments, whereas mitochondrial membrane potential (MMP), while susceptible to extreme hypo- and hypertonic environments, showed an ability to rebound from hypertonic stress when returned to isotonic conditions. The problem of chromatin relaxation is a major impediment to the successful cryopreservation of koala sperm.
Another important, but often ignored component in the development of semen preservation technology and artificial insemination, is an understanding of the normal flora and pathogens associated with the external genitalia, prepuce and semen. Prior to determining what antibiotics are required for semen diluents, it is first necessary to conduct culture and sensitivity tests of what organisms are present and what antibiotics they are sensitive to. We conducted a screening procedure with koala semen and were surprised by the diversity of microflora, describing a new Corynebacterium spp. in the process (Johnston et al. 1998). Once we understood what organisms were present (9 bacteria and two yeasts) and what antibiotics they were sensitive to (Penicillin G 1,000 IU/mL and gentamicin 100 μg/mL), we then had to ensure that the antibiotics were not spermicidal; this included antibiotic dose response studies on sperm parameters (motility) and validation of antibiotic action to prevent bacterial growth (Johnston et al. 1998).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree