Chapter 2 NEONATAL ALLOIMMUNE THROMBOCYTOPENIA Classification of immunodeficiencies Severe combined immunodeficiencies Complement component immunodeficiencies Advances in immunology and technology have contributed significantly to the understanding of fundamental concepts of disease, to the identification of preventive methods and to the evaluation of treatment responses. Nevertheless, many areas in the field of equine immunology await reagents and technical capabilities to expand our knowledge of the mechanisms involved in many infectious and non-infectious processes. A glossary of principal immunologic terminology is given in Box 2.1. There are two main categories of vaccines: 1. Dead or inactivated vaccines: these vaccines contain the whole inactive pathogen or selective antigenic elements of the pathogen; they induce mainly humoral immunity, however DNA vaccines may induce both humoral and cell-mediated immunity. (a) Chemically inactivated whole pathogen vaccines—immunogenic and easy to prepare vaccines that require adjuvants and regular boosters (b) Protein vaccines—naturally produced protein of organisms that are used as antigens; also require adjuvants (c) Recombinant subunits vaccines—synthetic production of antigenic peptides using expression systems to retain immunogenicity of the native protein; require the knowledge of a pathogen antigen that is important for immunity and adjuvants (d) DNA vaccines—the objective is to mimic natural immune response by the in vivo synthesis of antigenic proteins with both MHC class I and MHC class II antigen presentation, hence both cytotoxic and antibody responses. 2. Live vaccines: the attenuated organism is able to invade a cell and use its replication machinery. Therefore, the organism is processed by the endogenous pathway for MHC class I presentation to generate cytotoxic T cells (CTLs), and MHC class II presentation to generate long-lasting humoral responses. (a) Modified live vaccines (MLV) are modified organisms that have attenuated pathogenicity but still replicate in the vaccinated animal; the organism may be attenuated in cell culture by growing it in abnormal conditions, by the creation of temperature-sensitive mutants (random mutants that may reacquire virulence), by using recombinant DNA technology for predicted deletion mutation that cannot be reversed, or by using variant forms of the organism that affect other species. (b) Recombinant vector vaccines (RVV) are engineered bacteria or viruses (vaccinia virus) that become carriers (vectors) of selected antigenic peptides from other pathogens. The bacteria or virus infect cells in the host and carry a target peptide that is known to induce a protective response against the organism. A variant of this type of vaccine is a multivalent vaccine in which one modified-live virus of interest carries another virus peptide. The protocol for the immunization of foals has been revised because of a better understanding of the foal’s immune response to distinct vaccines in early life. Nevertheless, vaccination of foals is initiated around 3–6 mo of age, followed by one to two boosters 3–4 wk apart. In addition, vaccination of mares in late gestation against agents that are responsible for disease in early life is recommended in order to confer passive transfer of organism-specific immunoglobulins, e.g. rotavirus (q.v.) and Clostridium botulinum (q.v.). 1. Type I hypersensitivity reactions are mediated by antigen-specific IgE, mast cells, basophils and their mediators. Examples are urticaria (q.v.), insect-bite hypersensitivity (q.v.) and food allergy. 2. Type II hypersensitivity reactions involve autoantibodies IgM or IgG against specific (often self) cell surface or extracellular matrix antigens. Subsequently, there is opsonization and phagocytosis of these cells and complement- and/or Fc receptor-mediated cell destruction. Examples include autoimmune hemolytic anemia (q.v.), thrombocytopenia (q.v.), incompatible blood transfusions, pemphigus foliaceus (q.v.) and drug hypersensitivity (q.v.). 3. Type III hypersensitivity reactions are promoted by the random deposition of immunocomplexes of circulating antigens (self or foreign) and IgM or IgG antibodies in blood vessels, with subsequent complement- and Fc receptor-mediated recruitment and activation of leukocytes and vasculitis. Examples include serum sickness (antisera passive immunization) (q.v.), glomerulonephritis (q.v.) and purpura hemorrhagica (q.v.). 4. Type IV hypersensitivity reactions do not involve antibodies and are mediated by sensitized CD4+ T cells (Th1 response) and CD8+ T cells (direct cytotoxic effect), which induce infiltration of macrophages and inflammation mediated by cytokines. An example is contact dermatitis (q.v.). Anaphylaxis is a severe form of immediate hypersensitivity that manifests within minutes following exposure to an allergen (e.g. insect bite/sting, drugs or vaccines, food). The hypersensitivity reaction involves a primary exposure to an antigen, CD4+ T cell cytokine-mediated activation of B cells for the production of IgE, and the binding of IgE to receptors on mast cells and basophils. Upon a successive exposure to the sensitizing antigen, its cross-linking with IgE triggers the release of mediators in cytoplasmic granules. These mediators (histamine, tryptase, leukotriene [LT] C4, prostaglandin [PG] D2, tissue necrosis factor [TNF]) cause early effects of vascular permeability and dilatation, and late effects of inflammation that last approximately 24 h. Anaphylactic reactions can be uniphasic, biphasic or protracted. In the uniphasic reaction, clinical signs resolve within hours. In the biphasic reaction, there is a recurrence of the anaphylactic signs any time between 1 and 30 h after the initial remission. In some cases, the severity of the biphasic reaction is comparable to and often involves the same clinical signs and body systems as the initial reaction. In human patients, delay in the administration of epinephrine is associated with an increase in biphasic reactions and anaphylactic death. It is not clear in human medicine whether steroid therapy prevents biphasic reactions although its anti-inflammatory properties could counter the delayed inflammatory response of anaphylaxis. Sweet itch or insect-bite hypersensitivity (q.v.) is a seasonally recurrent hypersensitivity skin reaction to antigens in the saliva of Culicoides species characterized by widely distributed pruritic crusting dermatitis. Following antigen exposure, eosinophils are recruited to the affected skin in response to histamine, platelet-activating factor (PAF) and eotaxin. Eosinophils may contribute to the disease with the release of proteases and inflammatory mediators. The failure of the anti-idiotype control mechanism of antibody production may allow the production of autoantibodies. In addition, molecular mimicry of microbes and self-epitopes may result in immune responses that overcome immunologic tolerance and lead to tissue injury. Exposure of auto-antigens present in systems that are not normally visited by lymphocytes (e.g. a breakdown of the blood–brain barrier in the central nervous system) or the development of new epitopes on normal proteins may stimulate an immune response. Immune-mediated thrombocytopenia results either from the production and binding of immunoglobulins to platelets or megakaryocyte antigen surface (primary or idiopathic thrombocytopenia), or from the binding of immunocomplexes (antibodies against microorganisms, or a drug hapten) to the Fc receptors on platelets (secondary thrombocytopenia). In addition, platelet-bound IgM may fix complement. The antibody- or antibody– complement-coated platelets are non-specifically removed from the circulation by the reticuloendothelial system (macrophages in the spleen and liver that phagocytose the platelets via their Fc and complement receptors). The mechanism for autoantibody production may involve auto-reactive B cell clones that are stimulated during an immune response to infectious organisms, dysfunction in CD4+ T cell regulation of B cell response, antigenic mimicry and altered anti-idiotypic regulation of antibody production.
The immune system
EQUINE IMMUNOLOGY
INTRODUCTION
VACCINATION
Types of vaccines
Vaccination programs
HYPERSENSITIVITY REACTIONS
TYPES OF REACTION
ANAPHYLAXIS AND ANAPHYLACTOID REACTIONS
ALLERGY OR ATOPY
AUTOIMMUNITY
Autoimmune diseases
Immune-mediated thrombocytopenia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The immune system
Only gold members can continue reading. Log In or Register to continue
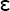 heavy chain on mast cells present in tissues or circulating basophils. Upon successive exposure, the allergen binds to the cell-associated IgE, and the cross-linking with other IgE molecules and Fc receptors triggers the release of mediators present in the cytoplasmic granules.
heavy chain on mast cells present in tissues or circulating basophils. Upon successive exposure, the allergen binds to the cell-associated IgE, and the cross-linking with other IgE molecules and Fc receptors triggers the release of mediators present in the cytoplasmic granules.