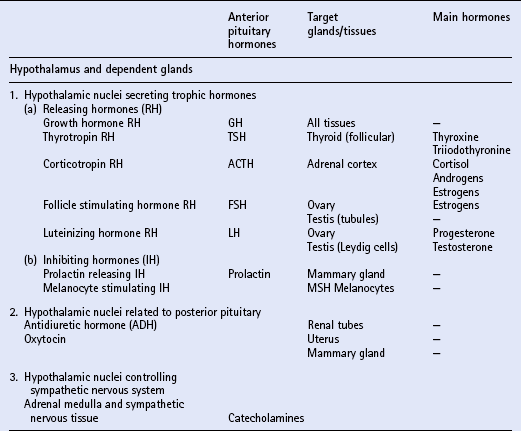
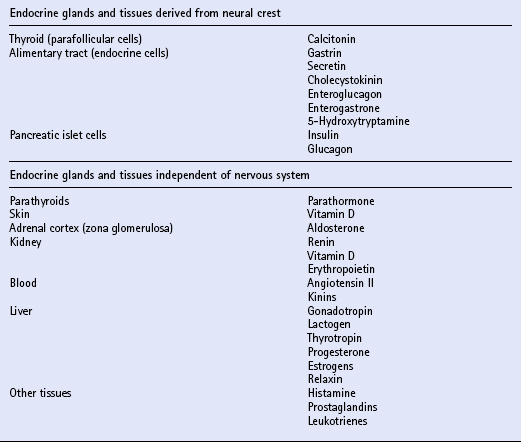
Chapter 11 Much of the endocrine system is related developmentally, anatomically or functionally to the nervous system. The hypothalamus is part of the brain and is a most important part of the endocrine system. It controls directly the adenohypophysis (anterior pituitary gland), and indirectly (via the pituitary) several other glands, including the adrenal cortex, the gonads and the major part of the thyroid. Part of the hypothalamus extends into the neurohypophysis (posterior pituitary) to form a single anatomic and functional unit. Nuclei within the hypothalamus control the sympathetic nervous system, which includes the adrenal medulla. Another group of endocrine tissues are derived embryologically from the neural crest, and are present as cells or clusters of cells within other organs; these include the parafollicular cells of the thyroid, the endocrine cells of the alimentary tract and the islet cells of the pancreas. Finally, there is a group of endocrine cells and tissues that is apparently independent of the nervous system; this includes the parathyroids, part of the adrenal cortex, and parts of other organs, tissues and blood. It is often said that the alimentary tract represents “the largest endocrine organ of the body”, and the complex endocrine and neural enteric systems have essential roles in the coordination of gut function, including motility, mucosal transport and local blood flow. The principal hormones and their sites of actions are summarized in Table 11.1. The equine pituitary gland measures approximately 2 cm × 1 cm and weighs from 1 to 3g. The terms anterior and posterior lobes are anatomically incorrect in horses, since the neurohypophysis (equivalent to the posterior lobe in primates) is embedded in and lies dorsal to the adenohypophysis (equivalent to the anterior lobe of primates). The adenohypophysis (80–85% of the total weight of the gland) is composed of endocrine cells derived from ectoderm. The neurohypophysis (15–20% of the total weight of the gland) consists of modified glial cells and axonal processes extending from nerve cell bodies in the supraoptic and paraventricular nuclei of the hypothalamus. Diagnosis may be achieved by using a water deprivation test and ADH response test. In the water deprivation test, access to all feed and water is denied and the bladder is emptied by catheterization. Urine specific gravity, body weight, blood urea and creatinine levels are monitored every 4 h for a maximum of 20 h. Water deprivation should be terminated if urine specific gravity rises >1.020, or if blood urea or creatinine levels increase above normal, or if there is clinical dehydration or a loss of more than 5% body weight. Horses in which urine specific gravity fails to exceed 1.020 during 20 h water deprivation are deficient in, or lack sensitivity to ADH. The ADH response test is performed by the IM injection of 40–60U pitressin (q.v.) following bladder evacuation and recording of urine specific gravity. Urine samples are collected 8, 12, 16 and 20 h post injection, and should exhibit maximal concentrations. Restoration of the ability to concentrate urine after ADH administration in an animal that failed to concentrate urine after water deprivation confirms central diabetes insipidus. The most commonly recognized abnormality of the adrenal cortex in the adult horse is hyperadrenocorticalism (Cushing’s disease, q.v.) secondary to pituitary pars intermedia dysfunction. Hypoadrenocorticalism secondary to synthetic steroid administration also occurs, but there are few well-documented cases. Laboratory confirmation of adrenal insufficiency depends on an abnormal response to ACTH challenge; this may be performed using either ACTH gel or cosyntropin (synthetic ACTH). The test should be performed early in the morning. The protocols are summarized in Box 11.1. Treatment of adrenal insufficiency includes rest and steroid supplementation. Cases of equine Cushing’s disease may be presented for investigation of a fairly diverse range of complaints, including lethargy, dullness, weight loss, excessive thirst, chronic infections, abnormal haircoat, persistent sweating and chronic, refractory laminitis (q.v.). The classic clinical sign of Cushing’s disease is generally considered to be hirsutism (a long, curly haircoat that fails to shed). Despite the characteristic appearance of “classical” cushingoid ponies or horses, it is not uncommon for owners to have accepted many, or all, of these features as normal aging processes. Clinically, the most distinctive feature of cases of equine Cushing’s disease is the abnormal hair growth pattern which presents as an extremely long haircoat (hirsutism), frequently with a very curly appearance, and which is often retained for prolonged periods including into the summer months. During the first few years of the disease, the abnormal haircoat may be restricted to the lower jaw, base of neck and palmar/plantar aspects of the distal limbs. Later, generalized hirsutism may develop, and in some cases a dark haircoat may turn lighter in color. In some animals, the haircoat may be found to be matted and wet due to persistent sweating (hyperhidrosis) unrelated to exercise. The pathogenesis of hirsutism and hyperhidrosis (q.v.) is unclear but it has been suggested that it may be related to elevated POMC peptides or possibly due to the physical effect of the tumor causing pressure on the adjacent hypothalamus, thereby affecting the thermoregulatory center. Adrenal cortical dysfunction may be assessed by the following: 1. Resting plasma levels of cortisol 2. Diurnal rhythm of plasma cortisol 3. Adrenocorticotropin stimulation test 4. Dexamethasone suppression test (DST) 5. Combined DST/ACTH stimulation test 6. Urinary corticoid-to-creatinine ratio Several different protocols for the DST have been proposed, and two are summarized in Box 11.2. The overnight protocol is useful as a screening test, but the standard protocol allows more detailed assessment of the degree of loss of pituitary function.
The endocrine system
INTRODUCTION
THE PITUITARY GLAND
INTRODUCTION
DIABETES INSIPIDUS
THE ADRENAL CORTEX
INTRODUCTION
ADRENAL EXHAUSTION AND THE ACTH STIMULATION TEST
CUSHING’S DISEASE
Clinical signs
Diagnosis
Dexamethasone suppression test
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The endocrine system
Only gold members can continue reading. Log In or Register to continue