Fig. 5.1.
Time taken to deplete a female Sprague–Dawley rat of 25OHD3 (•) compared with a control dam (∘) over the same pre-breeding period (mean ± SD). After 6 weeks on a diet deficient in cholecalciferol (119266; Dyets, PA, USA), 25OHD3 is virtually absent.
3.2 Preparation of the DVD-Deficient and Control Offspring
At this 10-week period, both vitamin D-deplete females and control females are mated with vitamin D normal males. Males are placed in with the females for 7 days. For experiments in embryos or fetuses where gestational time is required, dams are plug checked and vaginal smears are taken to ensure sperm presence once a day for the first 5 days (or until sufficient dams are confirmed). It is plausible that vitamin D-deficient females could absorb some vitamin D through grooming the vitamin D-replete male during breeding. We cannot rule this possibility out; however, to date we have not observed any increase in vitamin D levels in DVD-deficient females post-mating. This breeding protocol results in approximately 67% pregnancy rates independent of diet. Prospective pregnant dams remain on their respective diets throughout gestation and are housed in their same groups of four, until embryonic day 20 (E20), when they were housed individually and provided with nesting materials. When neonates are required, expectant dams are checked twice a day, beginning with the morning of E21, until litters are born. The first appearance of pups was recorded within 12 h of birth, and this day is referred to as postnatal day 0 (P0). On the day dams litter, both control and deplete dams are placed on the casein vitamin D-containing diet (#110700) and they remain under the same lighting conditions until the pups are weaned, at which time they are transferred to a separate animal holding room with standard fluorescent lighting and the dam is culled.
Litter sizes of between 8 and 18 are considered normal for Sprague–Dawley rats (31). At birth, litters of size less than 8 or greater than 18 are rejected (see Note 8). Both control and vitamin D-deficient dams and their respective offspring remain on the control diet (diet #110700) till weaning. In pups, 25OHD3 levels return to control levels by weaning (Fig. 5.2). All pups are weaned at 21 days into same sex groups of no specific number and all offspring are placed on a standard cereal-based rat chow that contains vitamin D (diet #119266). At 4 weeks of age, animals are split into groups of 2, 3, or 4, and housed in open-top wire cages, with no additional environmental enrichment. All animals remain under these conditions until behavioral testing (5, 10, or 20 weeks) after which the brain is analyzed for gross or cellular anatomy or protein or mRNA expression.
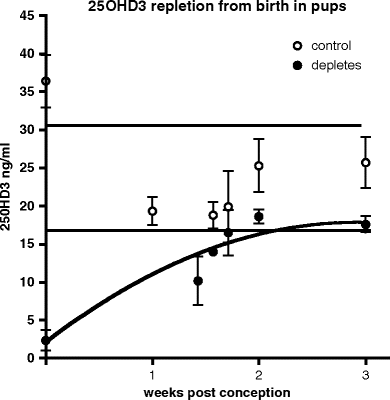

Fig. 5.2.
Time taken to replete a vitamin D-deficient dam (•) compared with a control dam (∘) over the same postnatal period (mean ± SD). 25OHD3 levels in vitamin D-deficient dams return to control levels 2 weeks after vitamin D is reintroduced in the diet. Horizontal lines indicate 25OHD3 concentration range (mean ± 1 SD) in control dams over this same postnatal period.
Vitamin D deficiency had no effect on dam or offspring weight gain either during pregnancy or after conception (Table 5.1). Serum calcium and phosphate measures were also normal in vitamin D-deficient dams despite a slight elevation in PTH across the period of pregnancy (Table 5.2). Rates of pregnancy and fecundity were also unaltered by maternal vitamin D depletion (Table 5.3). These findings conflict with other more drastic models of maternal vitamin D deficiency (see below).
Table 5.1
Vitamin D deficiency had no effect on dam or offspring weight gain either during pregnancy or after conception
Time | Control dam weight (g) | Vitamin D–deplete dam weight (g) | Control offspring weight, male (g) | DVD-deficient offspring weight, male (g) | Control offspring weight, female (g) | DVD-deficient offspring weight, female (g) |
|---|---|---|---|---|---|---|
Conception | 269±12 | 258±9 | ||||
E7 | 281±6 | 294±16 | ||||
E14 | 308±4 | 328±16 | ||||
P0 | 298±5 | 321±14 | 6.7±0.8 | 6.6±0.9 | 6.4±0.8 | 6.5±0.7 |
P7 | 338±7 | 337±10 | 21.5±1.9 | 21.9±1.4 | 20.9±1.1 | 20.8±1.3 |
P14 | 335±6 | 339±11 | 42.7±2.2 | 43.7±5.6 | 42.3±2.9 | 42.8±4.1 |
P21 | 328±6 | 325±6 | 61.4±3.0 | 64.7±8.8 | 60.4±4.6 | 61.6±6.0 |
P35 | 184.9±12.6 | 178.4±11.6 | 147.6±7.6 | 139.7±8.8 | ||
P70 | 461.1±38.6 | 475.8±43.2 | 265±20.9 | 264.3±30.5 |
Table 5.3
Rates of pregnancy and fecundity are unaltered by maternal vitamin D depletion
Measure | Control | DVD deficient |
|---|---|---|
%Pregnancies | 67.8 | 67 |
Average litter size | 11.4 | 11.8 |
Male/female ratio | 1.1 | 1.0 |
Litters <8 pups | 7.2% | 6.4% |
Litters >16 pups | 3.0% | 0.8% |
Table 5.2
Vitamin D deficiency did not affect serum calcium or phosphate at any stage of pregnancy. PTH levels were however elevated
Time | Ca2+ (mM) | PO4 (mM) | PTH (pg/ml) | |||
|---|---|---|---|---|---|---|
Control dam | Vitamin D-deficient dam | Control dam | Vitamin D-deficient dam | Control dam | Vitamin D-deficient dam | |
Pre-conception | 2.92±0.02 | 2.88±0.02 | 2.68±0.18 | 2.51±0.21 | 177.4±21.3 | 350.6±59.5* |
E7 | 2.86±0.04 | 3.01±0.03 | 2.20±0.14 | 2.47±0.12 | 98.5±20.8 | 280.5±55.8* |
E14 | 2.94±0.06 | 2.95±0.03 | 2.52±0.13 | 2.57±0.15 | 75.3±22.8 | 189.5±73.1‡ |
P0 | 3.38±0.07 | 3.25±0.08 | 2.21±0.29 | 1.82±0.27 | 233.7±104.0 | 725.2±191.2‡ |
The levels of 25OHD3 and 1,25OH2D3 in pups at birth reflect those seen in dams during pregnancy (25). DVD-deficient newborns are also normocalcemic (i.e., neither the dams nor their offspring have the rickets-like phenotype that would result in more chronic vitamin D depletion) (see Note 9). Observation of the offspring from birth to weaning indicated that maternal vitamin D depletion did not affect the progression of normal development or physical maturity. For instance, there were no significant differences between dietary groups on physical maturity scores (eye and ear opening, ear unfolding, fur development and teeth protrusion, self-righting reflex, and posture or stepping activity) (25). All the above physical and endocrine measures were also normal at the time of behavioral testing at either 10 or 20 weeks.
4 Other Models of Maternal Vitamin D Deficiency and General Measures of Pup Health
We have considered the effects of varying the duration of maternal vitamin D deficiency in the development of this model. We have examined both a shorter period of vitamin D depletion (e.g., gestation only) and two longer periods extending DVD deficiency (e.g., until weaning and throughout adulthood). Although gestational deficiency was also sufficient to produce important behavioral deficits in the offspring (e.g., NMDA antagonist-induced hyperlocomotion) (25), the level of the active form of the hormone 1,25OH2D3 in maternal animals was still well within control levels despite profoundly deficient levels of 25OHD3 (25). Extending maternal vitamin D deficiency until weaning produces changes in gross brain architecture consistent with schizophrenia (22); however, deficiency extended into the period of rearing increases possible associated physiological abnormalities such as hypocalcaemia, maternal weight loss, and reduced fertility (32). Lifelong vitamin D depletion apart from being completely non-physiological produced hypocalcaemia and associated cardio-vascular and kidney abnormalities (33) and was therefore rejected.
We have consistently shown that this model produces no gross abnormalities in birth outcomes, growth rates, or calcium status in either the vitamin D-deficient dams or DVD-deficient pups. However, other models of maternal vitamin D deficiency have not been so benign. Studies in the early 1980s reported that if female rats were kept vitamin D deficient for ≥90 days prior to mating, and during the period of gestation and rearing, then maternal growth and fecundity was reduced (34–36). Hypocalcemia in dams was also prevalent, but not universal (37). Curiously, where assessed, it appeared that in these earlier studies, pups appeared to be calcium normal. It has been suggested that fertility issues in the long-term vitamin D-depleted dams were secondary to low serum calcium and phosphorus rather than vitamin D deficiency per se (38).
The duration of maternal vitamin D depletion prior to mating used in our DVD model (42 days) is insufficient to affect maternal calcium and has no adverse effects on fertility, fecundity, or various measures of pup growth. However, this issue would appear to be not completely resolved with a recent study by a Japanese group showing that in female rats depleted in a similar fashion to that used here, both the gravid Sprague–Dawley dams and their offspring were hypocalcemic and fetuses were growth restricted (39). We advise those establishing the DVD-deficient model to first determine that their conditions do not have any adverse effects on either serum calcium levels or the aforementioned indices of maternal or fetal growth.
5 Conclusions
Most of our published data have been generated from offspring who experience a transient gestational period of vitamin D deficiency. Since our first studies were published (19), we have refined certain experimental factors such as lighting, litter size, and maternal calcium supplementation: We remain interested in what impact variations in the duration of developmental vitamin D depletion would have for brain development and function. We are also interested in the application of this model in other experimental animals such as wild-type (40) and transgenic mice. Other models could also be employed that, although having less face validity for the environmental nutrient deficiency being studied, could also reveal much about how vitamin D affects brain development. For example, studies that allow prolonged but less severe hypovitaminosis D also warrant inspection. Additionally, after birth, maternal vitamin D deficiency could be more rapidly reversed than the simple dietary intervention used here either with injections of the active hormone 1,25(OH)2D3 or by cross-fostering DVD-deficient offspring to control vitamin D normal dams. However, these models would require a substantial amount of preliminary studies to establish the correct drug/dosage/dosing interval prior to any consideration of their suitability for investigation.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


