CHAPTER 45 Systemic Clostridial Infections
CLOSTRIDIAL MYONECROSIS
Etiology
Clostridial myonecrosis in horses is most often caused by infection with Clostridium perfringens,1,2 although sporadic cases have been described in association with other Clostridium species, including C. septicum,1,3,4 C. chauvoei,1,2,5 C. novyi,1,6 C. ramosum, C. sporogenes,1 and C. fallax.7 The majority of cases in the literature have been single-species infections, but mixed infections have been reported.1,3 These highly pathogenic clostridial organisms are gram-positive, spore-forming, anaerobic bacilli that can elaborate numerous potent exotoxins. Vegetative growth of these clostridial species is accompanied by production of dermonecrotizing and vasoactive toxins that lead to gas production, extensive tissue damage, and necrosis, as well as rapidly developing, life-threatening systemic toxemia.
Epidemiology
The prevalence of clostridial myonecrosis is low, and although the disease is sporadic, more cases appear to occur in certain regions. Many cases reported in the literature are from the northeastern1,2,4 and midwestern1,5,8 areas of the United States, but the disease may occur anywhere in the United States,3 Canada,9 Europe,10 and the Southern Hemisphere.7,11 Although species such as Clostridium perfringens can frequently be cultured from the environment and soil wherever livestock are found, the means by which spores or vegetative organisms gain access to areas of affected soft tissue is not fully understood. Some of the species involved, including the most frequently isolated species, C. perfringens, can also be found within the gastrointestinal tract, in both the vegetative and the spore form, and some strains may be regarded as commensals. Spores of some clostridia (e.g., C. sporogenes, C. histolyticum) can be found in healthy equine muscle tissue, which suggests that after creation of appropriate anaerobic conditions, these spores may vegetate and begin exponential growth.12 However, spores of species typically isolated from clinical cases of equine myonecrosis have not, as yet, been identified dormant within muscle tissue.
Most cases of equine myonecrosis temporally occur soon after parenteral injection of pharmacologic or biologic agents (≤48 hours) in the affected area of the body.1,2,9 The condition is more common in the cervical musculature,1–4,8,9 but occasional cases involving the gluteal muscles1,8,9 and rarely the caudal thigh musculature1 have been reported. Cervical and throatlatch lesions are sometimes encountered secondary to inadvertent perivascular leakage of pharmacologics intended for intravenous (IV) administration.1 Traumatic wounds have rarely been associated with the condition.1,9 A wide array of pharmacologic and biologic preparations have been incriminated as inciting causes of clostridial myonecrosis, including nonsteroidal antiinflammatories,1,2,4,8,9 antihistamines,1,2,9 multivitamins,1,2,9 antipyretics,1,7,13 dewormers,2,9,14 vaccines, diuretics,1 and synthetic prostaglandins.13 The most frequently reported pharmacologic agent associated with the development of clostridial myonecrosis is flunixin meglumine, with the most cases occurring in the cervical region. 1–11,13,14
Pathogenesis
Most information regarding the pathogenesis of clostridial myonecrosis comes from rodent models of infection. Because identical species of clostridia, specifically C. perfringens and C. septicum, are the most commonly recognized causes of human clostridial myonecrosis, it is reasonable to assume that etiologic-specific pathogenic factors are comparable. During vegetative growth of C. perfringens, the primary toxin implicated in the disease process is alpha (α) toxin, a dermonecrotic toxin with both phospholipase C and sphingomyelinase activity.15 The definitive role that α-toxin plays during myonecrosis has been well established using controlled vaccine protection studies in which mice immunized against the C-terminal domain of the α-toxin were protected against lethal intramuscular (IM) doses of the organism, whereas unvaccinated controls were not.16 Deletional mutants of C. perfringens in which the structural α-toxin gene has been removed are nonpathogenic, and virulence is restored by recombination with a plasmid expressing the wild-type gene.17 Other toxins that can be produced during vegetative growth by C. perfringens and other clostridial species include theta toxin (perfringolysin), kappa toxin (collagenase), and mu toxin (hyaluronidase).15,18 Although other exotoxins elaborated by C. perfringens (and also other species) have highly potent and pathogenic effects extracellularly, no compelling evidence exists that they are required for lethal disease, as is the alpha toxin.
Many of the signs of systemic toxemia, cardiovascular collapse, and multiorgan dysfunction observed clinically in horses with clostridial myonecrosis can similarly be explained by observations made in rodent and rabbit models of gas gangrene. Alpha toxin directly suppresses myocardial contractility in vivo,19 whereas theta toxin is a potent reducer of systemic vascular resistance.20 Theta toxin has demonstrable ability to dysregulate polymorphonuclear/endothelial cell interactions, promoting leukostasis and interfering with normal cellular host responses to tissue injury at the active site of infection.21
Clinical Findings
Horses with clostridial myonecrosis demonstrate rapid soft tissue swelling, subcutaneous and deeper soft tissue emphysema, and rapid toxemia that may progress to circulatory collapse and multiorgan failure over just a few hours.1–3 Clinicopathologic data from horses that have died acutely corroborate multiorgan failure and diffuse intravascular coagulation as two major pathologic processes that occur in terminally ill horses with clostridial myonecrosis.1,2,8 Some horses that develop clostridial myonecrosis have a recent history of colic, resulting in the administration of IM analgesics, with subsequent myonecrosis in the region of the injection.1,2,8,9 Some authors have postulated that colic may truly be a prodromal sign of equine clostridial myonecrosis,4 drawing comparisons with the nausea and abdominal pain that are early symptoms of clostridial myonecrosis in human patients.22,23
Occasionally, horses with clostridial myonecrosis will develop hemolytic anemia/crisis after several days to 1 or 2 weeks of therapy.1,24 This appears to be a distinct entity to the life-ending, diffuse intravascular coagulation that other horses develop in the early stages of the disease. It is not certain whether hemolytic events are a direct effect of the clostridial infection. Some clostridia (e.g., C. septicum) can elaborate one or more exotoxins with in vivo hemolytic activity.22,23 Alternatively, hemolysis may be a potential immunologic complication of penicillin or other drug therapy.
Diagnosis
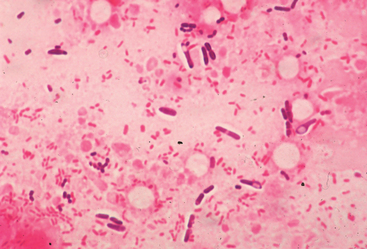
Clostridial myonecrosis may be presumptively diagnosed in any horse that develops acute-onset, rapidly progressive soft tissue swelling accompanied by emphysema in the area of a recent parenteral injection. Clostridial myonecrosis associated with penetrating traumatic wounds appears to represent a rare subset of cases.1,9 Acute-onset cellulitis without emphysema should be viewed as suspicious for the disease, but confirmed by cytologic evaluation and Gram stain before aggressive fasciotomy or debridement is performed (Fig. 45-1). Ultrasonography of areas of postinjection cellulitis should be performed to identify areas of deeper emphysema and gas production that may not yet be palpable in the immediate subcutis. Definitive etiologic confirmation can be achieved by Gram stain and anaerobic culture of fluid aspirates from an affected soft tissue area. Numerous, characteristic gram-positive rods, sometimes with endospores present, can be easily visualized on air-dried smears, particularly from the periphery of an area of soft tissue emphysema. Speciation after anaerobic culture by genetic or fluorescent antibody techniques provides definitive confirmation and may guide prognosis.1,25
Therapy
Higher survival rates are associated with aggressive combinations of medical and surgical treatment.1,2,4 Barotherapy has become a component of the approach to treatment of human cases of clostridial myonecrosis, but hyperbaric oxygen chambers are only beginning to become available for use in large animal veterinary medicine. Rapid therapeutic intervention with high doses of IV crystalline penicillins should be considered as soon as a presumptive diagnosis is made. Potassium penicillin at doses as high as 88,000 IU/kg every 2 hours (q2h) have been used, and conventional doses of 22,000 IU/kg q6h should be viewed as the minimum required dose, expense permitting.25 Oral metronidazole (25 mg/kg q6h) is often included in therapy but is unlikely to reach the high tissue levels that may be achieved with IV penicillin.
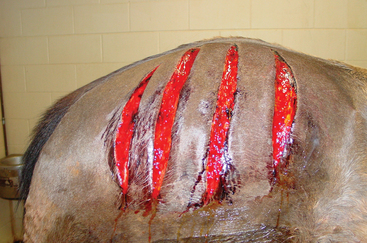
Intensive fluid, electrolyte, and cardiovascular support are indicated in the acute stages of clinical disease because dehydration and systemic toxemia can be life threatening at this time.1–3,25 Many affected horses become hypotensive, with diminished cardiac output and renal function, complicated by substantial myoglobin release and the potential for pigment nephropathy. Fenestration of affected soft tissues appears to be an important part of therapy, and clinicians are advised to be aggressive in incising areas of subcutaneous emphysema, extending the incisions into deeper tissues and adjoining areas of healthy tissue (Fig. 45-2). Because of the obtunded mentation of many affected horses and the rapid progression of tissue necrosis in affected areas, these procedures can usually be performed under sedation without the need for local anesthesia.25 Frequent reevaluation of the horse for signs of spreading emphysematous cellulitis, necessitating repeated incisions and debridement, is advised. Local infusion of penicillin into tissue at the margins of debrided muscle may have some benefit in limiting the spread of the infection.
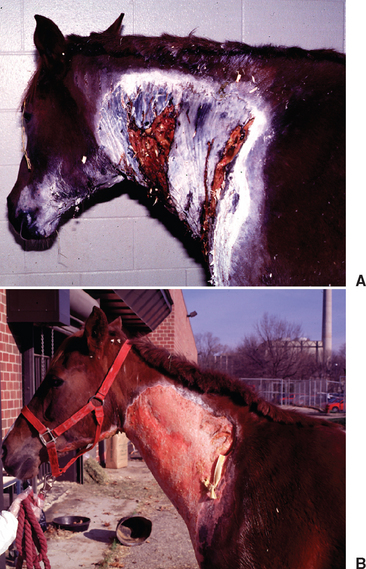
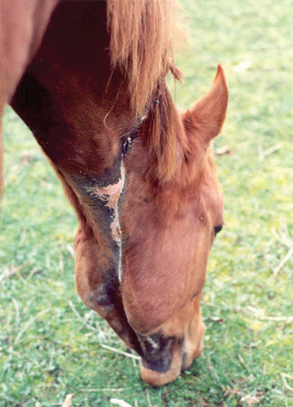
For horses that survive the acute stages of disease, prognosis will improve significantly. However, veterinarians should warn owners of the significant soft tissue and skin sloughing that will likely ensue over coming days to weeks (Fig. 45-3). Long-term wound care will often be needed, with many cases taking weeks to months before granulation and second-intention skin healing are complete. Cosmetically, some horses may heal with pigmentation changes and significant cicatrix formation (Fig. 45-4), but the visual appearance of healed wounds is often normal.25
Prognosis appears to vary according to the species of Clostridium involved. A much better prognosis is afforded to horses with soft tissue lesions associated with C. perfringens than to those with C. septicum or C. chauvoei infections.1 The largest retrospective study published to date reported an overall survival rate of 73% for horses with clostridial necrosis when they were treated with a combination of aggressive medical and surgical therapy in a referral hospital setting. Horses with C. perfringens infection had a survival rate of 81%.1 There was no gender predilection observed in that study, but the disease did appear disproportionately to affect Quarter Horses. Previous studies have demonstrated much higher case-fatality rates,2,8,9 and therefore clinicians and owners should be mindful of the need for prompt, aggressive, and potentially expensive therapy if horses are to have the best chance of survival. The majority of horses that die will succumb within the first few days.