CHAPTER 124 Surgery of the Reproductive Tract in Lamoids
Surgery of the reproductive tract in camelids includes most of the elective procedures as well as therapeutic surgical techniques considered for other domestic species. As the practice of theriogenology in lamoids has developed in recent years more information has become available on the specific considerations in reproductive surgeries in llamas and alpacas pertaining to anesthesia, surgical approach choices, and pre- and postoperative management. The objective of this chapter is to review the most important reproductive surgeries in lamoids, including laparoscopic techniques.
ANALGESIA AND ANESTHESIA
Local Anesthesia
The most common local anesthesia used in reproductive surgery is epidural anesthesia and local blocks. Although lumbosacral epidural anesthesia has been described by some authors, it is rarely used because it causes muscle weakness and presents the risk of cranial spread to T10-11. Epidural anesthesia is generally performed in the sacrococcygeal space or between the first and second coccygeal vertebrae and provides perineal analgesia without locomotion compromise. The intervertebral space is easily identified by palpation while moving the tail up and down. The skin is shaved and aseptically prepared. An 18-gauge, 1.5-inch needle is placed on dorsal midline with the bevel facing cranially and at a 60-degree angle to the tail head. The hub of the needle is filled with the solution and advanced until the solution is pulled into the epidural space.
Sedation and Tranquilization
Sedation of the patient is accomplished easily by administration of the drug of choice intramuscularly. The most commonly used sedatives in lamoids are the alpha-2 agonists xylazine and medetomidine1 (Table 124-1). Llamas are more sensitive to these sedatives than alpacas. Analgesia and sedation are increased by combination with butorphanol. Acepromazine is occasionally used for sedation but should be avoided in compromised animals because of its hypotensive effects and lack of reversal agents.
General Anesthesia
The most commonly used induction agent is ketamine. This drug has minimal effect on the cardiovascular system, does not depress reflexes, and provides some analgesia. The major problem is that it causes muscle rigidity and increased salivation. It is better used in combination with xylazine, xylazine and butorphanol, diazepam, or guaifenesin to improve muscle relaxation (see Table 124-1).
In pregnant animals (correction of uterine torsion or cesarean section), anesthesia is generally induced with propofol (3.5 mg/kg IV) and diazepam (0.5 mg/kg IV) or guaifenesin and maintained with isoflurane in oxygen.2 These drugs have minimal effect of the neonate. Propofol is rapidly redistributed and metabolized and diazepam has minimal placental transfer. Isoflurane is rapidly eliminated, making it well suited for cesarean section in most species.
In field practice and for rapid surgeries in healthy subjects the combination ketamine/xylazine is sufficient. The combination of butorphanol (0.1 mg/kg), xylazine (0.2 mg/kg), and ketamine (1.5 mg/kg IV) provides approximately 20 minutes of useful anesthesia for surgical procedures such as castration and testicular biopsy. I prefer to administer this combination IM. Ketamine may cause profound respiratory depression.
During recovery, the patient should be placed in sternal recumbency as soon as possible following surgery. The neck and head should be supported to prevent injury. Reversal agent should be administrated if the patient is still too sedated (see Table 124-1).
SURGERY OF THE MALE REPRODUCTIVE TRACT
Castration
Castration of the Normal Male
Much debate is ongoing about the most appropriate age for castration of lamoids. This is due to the effect of castration before puberty on bone growth and predisposition to arthritis. Castration at early age has been shown to delay closure of the long-bone physes, resulting in tall, straight-legged geldings and a predisposition to early onset of degenerative osteoarthritis. It is recommended that male lamoids be castrated no earlier than 15 months of age for alpacas and 18 months for llamas.3
Two techniques are used for castration of the male lamoid: scrotal and prescrotal. Prescrotal technique requires more time to complete, but is more esthetic and less painful.3,4
Scrotal technique.
This technique is performed on the male either in the standing position or in lateral recumbency.5,6 It is the author’s preference to use the standing procedures on llamas and lateral recumbency in alpacas as the latter tend to cush, which makes manipulation and surgery very difficult and presents a risk for contamination. For standing castration sedation and analgesia is obtained by using butorphanol alone with local scrotal and testicular infiltration of lidocaine5 or by administration of a combination of butorphanol and xylazine. Some practitioners prefer administration of an epidural, but this is generally not necessary. For recumbent patient castration a combination xylazine/ketamine or xylazine/ketamine/butorphanol is indicated. Local anesthesia of the scrotum is provided by injection of 1 to 2 ml of lidocaine 2% as a line block along the raphe median.
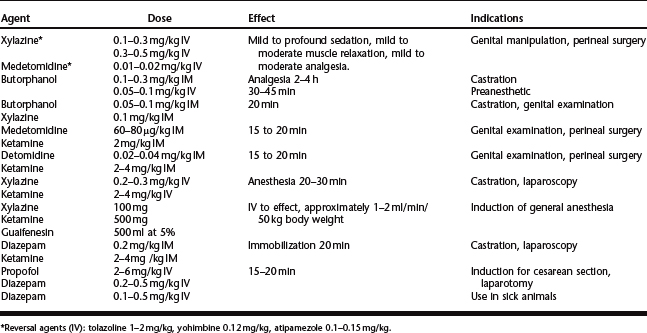
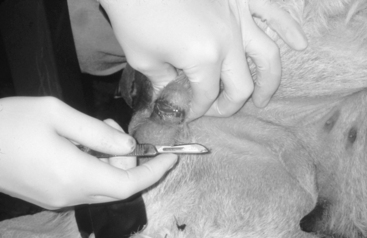
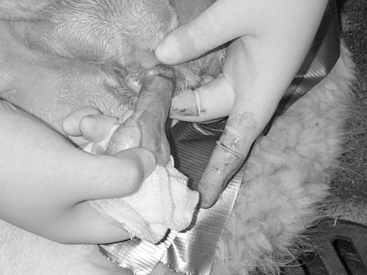
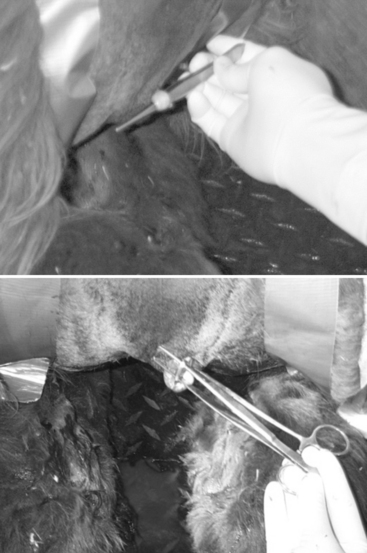
The technique is not different from that described for other species. The scrotum is prepared for aseptic surgery and an incision is made along the most ventral aspect of the scrotum by holding the testicle firmly into the scrotum to make the scrotal skin tight (Fig. 124-1). The extent of the incision is determined by the size of the testicles and is generally continued until the testicle and its envelopes protrude under pressure from the skin incision. The testicle is exteriorized and held with a towel clamp while the testicular cord is gently stripped from fat and connective tissue using a piece of gauze (Fig. 124-2). The testicle is removed using an emasculator (adult llamas) or after transfixation ligation of the spermatic cord with No. 0 chromic gut (llamas) or No. 2-0 polyglactin 910 (young llamas and alpacas) (Fig. 124-3).
Prescrotal technique.
This approach requires the animal to be in a dorsal recumbency position under general anesthesia.4 The animal is maintained in a frog-leg position by means of ropes or straps. Strict aseptic technique is critical to prevention of infection. The skin is incised on the ventral midline immediately cranial to the ventral base of the scrotum.
Castration of the Cryptorchid Male
Cryptorchidism is relatively rare in lamoids but has been reported in llamas, alpacas, and vicunas. There are very few reports on surgical removal of the retained testis. Two approaches can be utilized: parainguinal approach7 or a laparoscopic approach (see under laparoscopic surgery).
Vasectomy
Vasectomy is mainly used for the preparation of teasers for the induction of ovulation in camelidae for the purpose of scientific studies. However, because of its esthetic advantages some breeders may opt for this technique rather than castration to sterilize males that are undesirable for reproduction.3
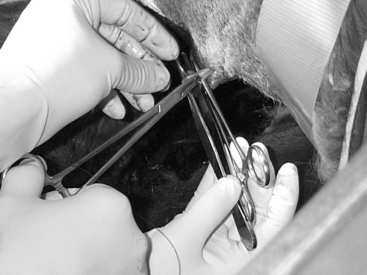
Vasectomy can be performed in the llama in a sitting or standing position after sedation, but the dorsal recumbency is the preferred position in alpacas. The scrotal skin is prepared by clipping and surgical scrubbing. Surgical drapes are placed around the scrotum. A 2- to 4-cm vertical scrotal skin incision is made slightly medial on the cranial surface of the neck of the spermatic cord. The spermatic cord is freed by blunt dissection and exteriorized with the help of hemostatic forceps (Fig. 124-4). The vas deferens can be easily identified by palpation or visually by its white color and the presence of adjacent vein and artery. The vas deferens is exteriorized using forceps or a spay hook through a small nick made in the vaginal tunic. A 3-cm portion of the vas deferens is removed after ligating each end (Fig. 124-5). The vaginal tunic does not need to be sutured. The skin is sutured, and the same procedure is repeated on the other side. Excised tissue should be submitted for histologic confirmation.
Another approach to vasectomy is to make the incision near the inguinal canal. This technique provides an easier identification of the vas deferens, particularly in individuals that have a short testicular cord. A subcuticular suture provides closure of the surgical site. Alternatively, a 2-cm segment of the ductus deferens may be excised via standard laparoscopy using a forceps scissors8 (see discussion of laparoscopic surgery).
SURGERY OF THE FEMALE REPRODUCTIVE TRACT
Cesarean Section
Cesarean section is indicated if vaginal delivery is impossible. The most common causes of dystocia requiring cesarean section include failure of cervical dilation, uterine torsion, and fetal malpresentation. In alpacas, delivery by cesarean section may be indicated even if the fetus is dead because of the difficulty of manipulation and procedures such as fetotomy. Damage to the cervix or uterus is more likely to occur when trying to force manipulation of the fetus because of inadequate space or cervical dilation. The decision to proceed with a cesarean section should be made relatively quickly in order to improve the chances of fetal and maternal survival. If the size of the dam precludes transvaginal palpation, immediate cesarean section should be chosen.9
Two approaches are utilized for cesarean section in lamoids: the ventral midline approach and the left paralumbar approaches.9,10
Ventral Midline Approach
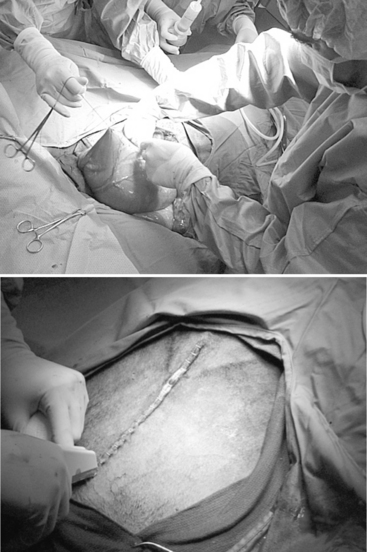
The ventral midline approach has been suggested as the preferred approach for alpacas and llamas. This allows simple access to the abdominal cavity and complete exteriorization of the uterus with minimal hemorrhage. Anesthesia is generally induced with propofol and diazepam (0.5 mg/kg IV) or guaifenesin. Although some have used halothane, maintenance of anesthesia is usually provided by isoflurane in oxygen.11 The patient is placed in dorsal recumbency and the ventral midline is prepared aseptically. A midline celiotomy incision (25 cm in alpacas and 35 to 40 cm in llamas) is made through the skin, subcutaneous fat, cutaneus trunci muscle, and linea alba from the cranial border of the mammary gland extending cranially (Fig. 124-6). The uterus is identified by direct palpation and exteriorized from the abdomen.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree