CHAPTER 28 Streptococcal Infections
OVERVIEW
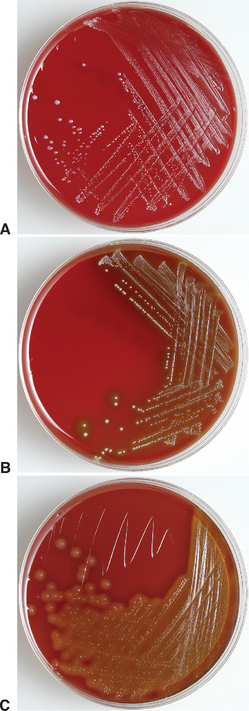
The streptococci are gram-positive, catalase-negative, facultative anaerobic, coccoid or ovoid bacteria. The numerous species of streptococci may be described on the basis of the nature and extent of their hemolytic activity as alpha-hemolytic (α-hemolytic), beta-hemolytic (β-hemolytic), and nonhemolytic species (Fig. 28-1). The most significant streptococcal pathogens in human and veterinary medicine are β-hemolytic, producing complete clearing of blood agar medium because of lysis of erythrocytes in the media. In α-hemolytic reactions, erythrocytes are not completely lysed, and growth is surrounded by greenish color of the agar resulting from streptococcal action on hemoglobin (see Chapter 27). Nonhemolytic streptococcal species have no effect on blood agar.1
In 1928 an American bacteriologist, Rebecca Lancefield, published a study of the chemical composition and antigenicity of hemolytic streptococci. In 1933 she described a classification of streptococci into groups according to their ability to induce production of antibodies that cause precipitation of streptococci from solution. These groups are now known as the Lancefield groups. The Lancefield group A streptococci, most notably Streptococcus pyogenes, are the most important streptococci in human medicine; in contrast, the group C streptococci S. equi subsp. zooepidemicus and S. equi subsp. equi assume greater importance in equine medicine. Although the Lancefield groupings remain phenotypically useful, it is now known that unrelated species of β-hemolytic streptococci may produce identical Lancefield antigens and that strains that are genetically related at the species level may have heterogenous Lancefield antigens.1 Molecular analysis of streptococcal organisms have revealed that the bacteria formerly classified as group D streptococci, or enterococci, are better classified in the separate genus Enterococcus (see Chapter 30) and the group N streptococci, or lactococci, should be classified in the genus Lactococcus.
This chapter discusses individually the major streptococcal pathogens of horses, including S. equi subsp. equi, S. equi subsp. zooepidemicus, and S. pneumoniae. A variety of other streptococcal infections have been described in horses as nonspecific commensal organisms or sporadic etiologic agents of disease. Notable among these reports for the severity of associated disease, streptococcal toxic shock was reported in a horse in which Streptococcus mitis was cultured from the blood.2
STREPTOCOCCUS EQUI SUBSP. EQUI
Corinne R. Sweeney, Peter J. Timoney, J. Richard Newton, and Melissa T. Hines*
Disease caused by S. equi subsp. equi infection in horses, commonly referred to as “strangles,” was described in early veterinary science literature and first reported by Jordanus Ruffus in 1251. Although its official name is S. equi subsp equi, there is compelling evidence that it is derived from an ancestral S. zooepidemicus as a “genovar” or “biovar” of the latter. The descriptive term S. equi is still appropriate based on its widespread usage in the scientific literature over the past century and is used in this discussion.
Etiology
Streptococcus equi is a gram-positive coccoid bacterium that typically appears in pairs or chains. Colonies on blood agar plates are mucoid, honey colored, and surrounded by a wide zone of beta hemolysis. Its colony morphology is identical to that of S. equi subsp. zooepidemicus (see later discussion), and it must be differentiated by its inability to ferment lactose and sorbitol. There is only one known antigenic type of S. equi, which may be a clone or biovar of the closely related S. equi subsp. zooepidemicus.3–5
Virulence factors for S. equi include a nonantigenic hyaluronic acid capsule, hyaluronidase, streptolysin S, streptokinase, immunoglobulin G (IgG) Fc-receptor proteins, pyrogenic exotoxins (e.g., SePE-I, SePE-H), peptidoglycan, and the antiphagocytic M protein (SeM).5 Evidence also exists for a leukocidal toxin.6 Virulent isolates of S. equi are almost always highly encapsulated, producing mucoid colonies, whereas avirulent isolates lack a capsule.7,8 The capsule appears to inhibit significantly the ability of neutrophils to bind, ingest, and kill the organism. It also facilitates the function of proteases and toxins within the organism and is required for the function of SeM.5 The oxygen-stable enzyme streptokinase S is responsible for the β-hemolysis observed with S. equi. Binding to erythrocytes results in formation of a transmembrane pore and irreversible osmotic lysis of the cell.9 At least four pyrogenic mitogens, SePE-H, SePE-I, SePE-K, and SePE-L, are expressed by S. equi.5 These molecules stimulate T cells nonspecifically to proliferate and release proinflammatory cytokines, resulting in an acute-phase response with high fever, neutrophilia, and hyperfibrinogenemia. These clinical responses may be neutralized by antibody generated during convalescence or by active immunization with each mitogen.10
The M proteins of streptococci are antiphagocytic, acid-resistant fibrillar molecules that project from the cell wall surface in an arrangement whereby two identical molecules are coiled around each other.5 The M protein of S. equi (SeM) is approximately 58 kilodaltons. Its antiphagocytic action is a result of binding of fibrinogen to the N-terminal half of the molecule and IgG to the central region.11–13 This interaction masks C3b-binding sites on the surface of the bacteria and inhibits the alternative C3 and classic C5 convertases.14 The M protein of S. equi is highly conserved with little variation in size or antigenicity between isolates, except for some isolates from long-term guttural pouch carriers that have in-frame deletions representing about 20% of the SeM gene.15,16 Loss of SeM expression by S. equi results in loss of virulence, but not infectivity, for ponies.17
Epidemiology
Transmission
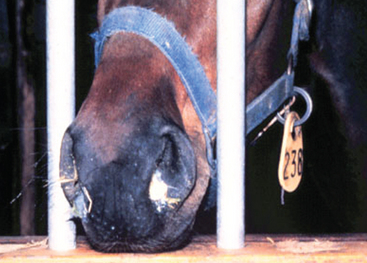
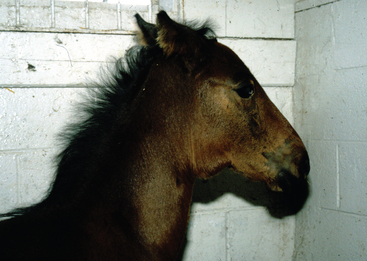
Purulent discharges from horses with active and recovering strangles are an important and easily recognizable source of new S. equi infections among susceptible horses (Fig. 28-2). Transmission of infection occurs when there is either direct or indirect transfer of S. equi within these purulent discharges between affected and susceptible horses. Direct transmission refers to horse-to-horse contacts, particularly through normal equine social behavior involving mutual head contact. Indirect transmission occurs through the sharing of contaminated housing, water sources, feed or feeding utensils, twitches, tack, and other less obvious fomites, such as the clothing and equipment of handlers, caretakers, farriers, and veterinarians, unless appropriate barrier precautions are in place to prevent spread of S. equi.
Inapparent Carrier Horses.
Transmission of S. equi that originates from outwardly healthy animals incubating the disease or that are inapparent carriers of the organism in their upper respiratory tract has been the focus of recent investigation.18–22 Normal nasal secretions are assumed to be the source of infection in these animals. Evidence indicates that a moderate proportion of horses continue to harbor S. equi for several weeks after clinical signs have disappeared, even though the organism is no longer detectable in most affected horses by 4 to 6 weeks after total recovery. In this situation the source of infection may not be readily apparent, and clinical signs may appear unexpectedly in animals with close contact to these carriers.
Horses that are fully recovered from the disease but continue to be infectious for prolonged periods through periodic shedding of S. equi are referred to as long-term, subclinical carriers and can be a source of infection for susceptible animals.18–22 Introductions of these animals to herds may be a source of new outbreaks, even in well-managed groups of horses. Adequate control for strangles cannot be achieved without recognition of this category of animal and implementation of appropriate methods for detection and treatment.
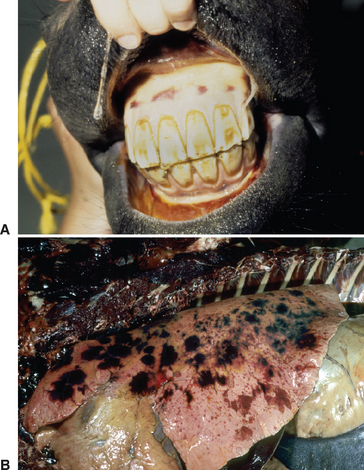
The best recognized site of carriage of S. equi among subclincially infected horses is the guttural pouch. This structure becomes infected in the early stages of disease, following rupture of the adjacent retropharyngeal lymph nodes through the floor of the pouch (Fig. 28-3). It is likely that short-lived guttural pouch empyema is the most frequent outcome of uncomplicated drainage of retropharyngeal lymph node abscessation. The carrier state develops in approximately 10% of affected animals, resulting in chronic empyema of the pouches. Should the purulent material persist in the guttural pouch, inspissation occurs with formation of discrete masses known as chondroids. Chondroids may occur singly or multiply, sometimes in very large numbers (Fig. 28-4). Chondroids formed after strangles can harbor S. equi, based on culture and by histologic demonstration of the organism on the surface of and lining the fissures within these structures (J.R. Newton, unpublished data). In some animals, guttural pouch empyema with S. equi infection may persist without clinical signs for many months or even years. Approximately 50% of horses with guttural pouch empyema cough sporadically, and some may have an intermittent unilateral nasal discharge.
Environmental Persistence of S. equi.
Currently, there is a lack of field-based proof for prolonged environmental persistence of S. equi. A suspension of bacteria survived for 63 days on wood at 2° C and for 48 days on glass or wood at 20° C. The absence of co-infection with normal environmental flora in this study might have prolonged the survival because S. equi is sensitive to bacteriocins produced by environmental bacteria.23 Its survival time is decreased in the presence of other soil-borne flora.24
Pathogenesis
S. equi enters through the mouth or nose and attaches to cells in the crypt of the lingual and palatine tonsils, as well as to the follicular-associated epithelium of the pharyngeal and tubal tonsils. A few hours after infection, the organism is difficult to detect on the mucosal surface but is visible within cells of the epithelium and subepithelial follicles. Translocation occurs in a few hours to the mandibular and suprapharyngeal lymph nodes that drain the pharyngeal and tonsil region. Failure of neutrophils to phagocytose and kill the streptococci appears to be caused by a combination of the hyaluronic acid capsule, antiphagocytic SeM protein, Mac protein, and other undetermined antiphagocytic factors released by the organism.5,24 This culminates in accumulation of many extracellular streptococci in the form of long chains surrounded by large numbers of degenerating neutrophils. Bacterial enzymes such as streptolysin and streptokinase may contribute to abscess formation by damaging cell membranes and activating plasminogen.
Although strangles predominantly involves the upper airways, including the guttural pouches and associated lymph nodes, metastasis to other locations occasionally occurs. Spread may be hematogenous or through lymphatic channels, which results in abscesses in lymph nodes and other organs of the thorax and abdomen. This form of the disease has been known as “bastard strangles.” Bacteremia occurs on days 6 to 12 in horses inoculated intranasally with virulent S. equi.25
Nasal shedding of S. equi usually begins after a latent period of 4 to 14 days and ceases between 3 and 7 weeks after resolution of the acute phase of disease.5,24 In some horses, shedding persists beyond this period; these animals may continue to harbor S. equi in their guttural pouches or other areas of the upper respiratory tract for months or years.18,20
Approximately 75% of horses develop solid immunity to S. equi after recovery from infection.8 The other 25% of infected horses are susceptible to reinfection within months, probably because of a failure to produce or maintain adequate concentrations of appropriate mucosal and systemic antibodies.5 Strong SeM-specific mucosal immunoglobulin A (IgA) and IgGb responses occur after infection in most horses. The development of mucosal and serum antibody responses are independent; local responses require local antigenic stimulation.8 Local anamnestic responses contribute to protection if the horse is reexposed. Older horses with residual immunity have limited susceptibility to S. equi and often develop a mild form of strangles termed “catarrhal strangles.”24 Despite the lack of classic clinical signs of strangles, these horses will shed virulent S. equi in nasal secretions in sufficient numbers to be a serious threat of transmission of disease to younger, more susceptible horses.
Colostrum from mares that have recovered from strangles contains IgGb and IgA with specificities similar to those found in the nasopharyngeal mucus of convalescent horses.26 These antibodies recirculate to the nasopharyngeal mucosa after a foal ingests colostrum and provide resistance to infection until approximately the time of weaning.
Although it is widely believed that the SeM protein is a major protective antigen, it is clear that other antigens are also important. An SeM-negative mutant of S. equi used as an intranasal vaccine protected horses against subsequent experimental challenge with virulent bacteria.17
Several aspects of S. equi pathogenesis are important to consider in the design and implementation of strangles control programs (Box 28-1).
Box 28-1 Aspects of Pathogenesis Important in Control and Prevention of Strangles
Clinical Findings
Strangles is characterized by abrupt onset of fever followed by upper respiratory tract catarrh, as evidenced by mucopurulent nasal discharge (see Fig. 28-2) and acute swelling, with subsequent abscess formation in submandibular and retropharyngeal lymph nodes (Fig. 28-5). The term strangles was coined because affected horses sometimes were suffocated by enlarged lymph nodes that obstructed the airway. Severity of disease varies greatly depending on the immune status of the animal. Older horses often exhibit a milder form of the disease characterized by nasal discharge, small abscesses, and rapid resolution of disease, whereas younger horses are more likely to develop severe lymph node abscesses that subsequently open and drain (Fig. 28-6).
Lymphadenopathy is a major clinical sign (see Figs. 28-5 and 28-6). The submandibular and retropharyngeal lymph nodes are involved with about equal frequency in S. equi infections and become swollen and painful about 1 week after infection. The first sign of lymphadenopathy is often hot, diffuse, painful edema. Serum may then ooze from the overlying skin for several days, as the lymph node abscesses mature before rupturing to drain tenacious creamy pus, which does not have a foul odor. Other lymph nodes of the rostral neck (parotid, cranial cervical, and retropharyngeal) are also frequently involved and may abscess. Retropharyngeal lymph nodes may drain into and cause empyema of the guttural pouch. Natural drainage of these deeper abscesses to the skin may take several days or weeks, and the swelling can exert pressure on the pharynx, larynx, trachea, and esophagus, causing severe dyspnea, stridor, and dysphagia. Retropharyngeal lymph node abscessation is not always associated with swelling that can be appreciated externally. Periorbital abscesses can cause marked swelling of the eyelids. Abscesses of the lymph nodes at the thoracic inlet can cause severe tracheal compression, asphyxia, and death. Coughing is not a significant feature in most cases, although some horses develop a soft, moist cough that becomes more productive and increasingly severe as the disease progresses. Squeezing the larynx will often cause marked pain, stridor, and gagging, followed by a retching cough and extended neck position when the neck is released. Expulsion of large quantities of pus from the nose or mouth with coughing usually indicates empyema of the guttural pouch.
Manifestations Associated with Severe Lymph Node Enlargement
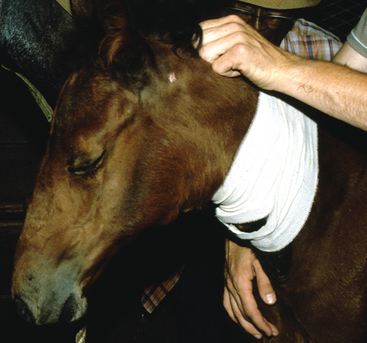
Abscessation, particularly of the retropharyngeal lymph nodes, may result in obstruction of the upper respiratory tract. The enlarged lymph nodes may compress the pharynx, larynx, or trachea, necessitating a tracheostomy in severe cases (Fig. 28-7). Temporary laryngeal hemiplegia, resulting from damage to the recurrent laryngeal nerve from enlargement of either the retropharyngeal or the anterior cervical lymph nodes, may also contribute to dyspnea. Four of 15 horses with complicated strangles had upper respiratory tract obstruction requiring tracheostomy, and death was attributed to the obstruction in two of these horses.27 Dysphagia may also occur as a result of lymph node enlargement or guttural pouch empyema.
Complications Associated with Metastatic Spread of Infection
Internal Infection.
Approximately 20% of horses with strangles experience some type of complication, and the presence of complications greatly increases the case-fatality rate.27,28 Of 74 horses with strangles on a 235 horse farm, 15 (20.3%) had complications; case-fatality rate in horses with complications was 40% compared with an overall case-fatality rate of 8.1%.27 Complications with strangles include spread of infection to parts of the body other than the head and neck, immune-mediated processes, and agalactia in mares.24
Common sites of infection include the lung, mesentery, liver, spleen, kidneys, and brain. Respiratory distress may be caused by tracheal compression resulting from enlargement of the cranial mediastinal lymph nodes. Suppurative bronchopneumonia is an important sequela of strangles. Of 15 horses with complications associated with strangles, five had pneumonia or pleuropneumonia, and three of five deaths were attributed to pneumonia, making this the most common complication resulting in death.27,29 In a previous study, 22 of 35 animals with complications (62%) died of pneumonia secondary to strangles.28
Another important complication of strangles is extension of infection to the sinuses or guttural pouches.27 In a general study of guttural pouch empyema, S. equi was isolated in 14 of 44 horses, and 5 of 74 horses with strangles had guttural pouch disease.29 Infection of the guttural pouch is of particular importance because the guttural pouch is the most common site for prolonged carriage of the organism.20,30 Horses with infection in the sinuses may also become carriers.
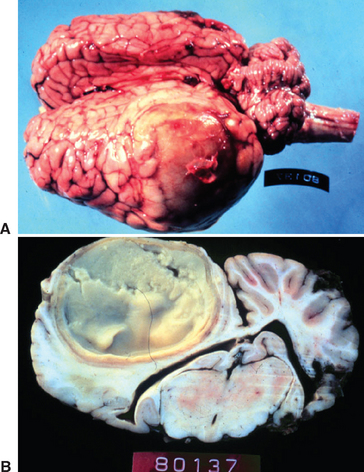
The prevalence of metastatic abscessation is generally low. However, in a recent study in which outbreaks of strangles on two farms were investigated, 7 of 25 (28%) developed metastatic abscessation.3124 Of these, euthanasia was performed in five horses, four of which had neurologic signs and confirmed cerebral abscesses (Fig. 28-8). The reason for the high incidence of complications, particularly neurologic disease, on these farms is unclear, but possible theories include a high infectious dose, the virulence of the strains involved, differences in host susceptibility, and other, unidentified factors.
It has been suggested that antimicrobial treatment after development of an abscess might contribute to metastasis, based on the theory that protein synthesis by the organism is altered by antimicrobial treatment. Further, a reduced immunogen level results in suboptimal immune response. Currently, however, no experimental or clinical data support the theory that antimicrobial treatment increases the prevalence of bastard strangles. In the study by Spoormakers et al,31 no antibiotics were used in any of the cases before complications were identified, yet the incidence of significant complications was high, and it is known that metastatic infection has occurred in other outbreaks where antibiotics have not been used.
Immune-Mediated Complications
Purpura Hemorrhagica.
Purpura hemorrhagica is an aseptic necrotizing vasculitis characterized primarily by edema and petechial or ecchymotic hemorrhage. Although the exact pathogenesis of purpura hemorrhagica is not fully understood, it appears to be a vasculitis caused by the deposition of immune complexes in blood vessel walls. Although often associated with S. equi infection, purpura may also occur in response to a number of different antigens. Of 53 horses with purpura, 17 were exposed to or infected with S. equi, and five were vaccinated with S. equi M protein; the remaining 31 cases either were associated with other organisms or had no known cause.32
The risk of developing purpura hemorrhagica after exposure to S. equi through infection or vaccination is not known. Four of 74 horses with strangles developed purpura, and all four were male yearlings that had been vaccinated with an M-protein vaccine, and all developed signs of purpura hemorrhagica within 2 to 6 days after the onset of strangles.27 A preexisting high serum antibody titer to S. equi antigens may predispose horses to the development of purpura. Studies have suggested an association between the development of purpura and antibodies of the IgA isotype. IgA titers to both M-like protein and culture supernatant proteins were higher in horses with purpura than in horses either recently infected with S. equi or with no known history of exposure.33 In addition, an increase in the IgG antibody titer coincided with the onset of clinical recovery in horses with purpura. Immune complexes with IgA and M-like proteins have been found in the sera of horses with purpura.34 The immunologic basis for the high concentrations of IgA and low concentrations of IgG during the acute stages of purpura is not understood. Some proposed explanations include uncontrolled expansion of B-cell populations that produce IgA, failure of IgA removal mechanisms, delayed production of IgG in response to a novel stimulus, defective or suppressed production of IgG, and neutralization or excess utilization of IgG.
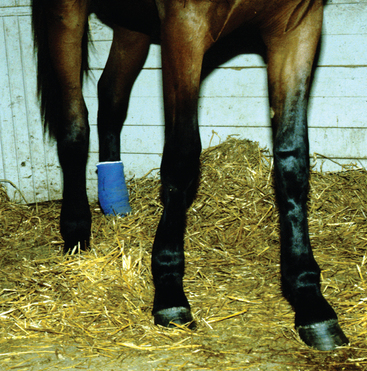
The severity of clinical signs seen with purpura varies from a mild, transient reaction to a severe, fatal disease.32 The typical clinical signs seen as a result of the vasculitis include subcutaneous edema, most frequently involving the head, limbs, and trunk (Fig. 28-9), and petechiation and ecchymoses of the mucous membranes (Fig. 28-10). Severe edema may result in oozing from the skin surfaces, and sloughing of the skin may occur (Fig. 28-11). In some horses the vasculitis may affect other sites, such as the gastrointestinal (GI) tract, lungs, and muscle, resulting in signs that include colic, respiratory difficulties, and muscle soreness.
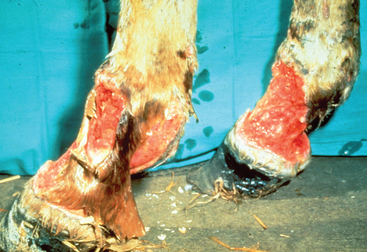
Fig. 28-11 Sloughing of skin on distal limbs of horse with purpura hemorrhagica.
(Courtesy Dr. Warwick Bayly.)
Leukocytoclastic vasculitis on histologic examination of skin is consistent with a diagnosis of purpura hemorrhagica. In those cases associated with S. equi infection, isolation of the organism and demonstration of elevated IgA and IgG titers to S. equi are also supportive.
Corticosteroids are the primary treatment for purpura. Generally, dexamethasone at 0.1 to 0.2 mg/kg, followed by a tapering-dose regimen, is used. In those cases where purpura is associated with active bacterial infection or the horse is considered at high risk of developing infection, appropriate antibiotic therapy is also indicated. Nonsteroidal antiinflammatory drugs (NSAIDs) may be of some benefit in some cases of purpura. Supportive care, including intravenous (IV) fluids, hydrotherapy, and bandaging, may also be indicated. The majority of the 53 horses with purpura in one report were treated for more than 7 days.32
Purpura hemorrhagica can be a serious complication of strangles. One of the four horses with purpura was euthanized because of the severity of the skin necrosis.27 Similarly, 3 of 22 horses with purpura secondary to exposure to S. equi did not survive.32
Myositis.
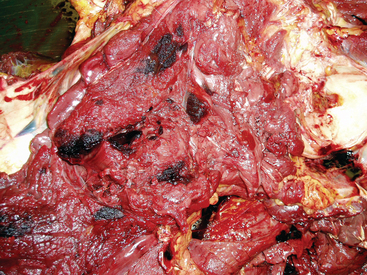
Two types of myopathy—muscle infarction and rhabdomyolysis with progressive atrophy—have been documented in horses after exposure to S. equi. These syndromes are presumed to be immune mediated, although through different mechanisms. The exact pathogenesis for the syndromes has not been fully elucidated, but muscle infarction is thought to result from an immune-mediated vasculitis, and rhabdomyolysis with progressive atrophy is believed to result from cross-reactivity between SeM and myosin. In a study of 25 horses with either strangles or purpura, eight had evidence of muscle abnormalities based on serum chemistries and histologic muscle lesions.35 Four of these horses had muscle infarctions, and the other four had rhabdomyolysis.
Muscle infarctions are most likely a manifestation of purpura hemorrhagica (Fig. 28-12). Many horses with purpura exhibit mild elevation in serum creatine kinase (CK) activity resulting from vasculitis within the muscle and mild muscle necrosis. Titers of SeM-specific antibody may exceed 1:6400. Some horses develop a severe vasculopathy characterized by infarction of skeletal muscle, skin, GI tract, and lungs.35 These horses present with muscle stiffness, lameness, and increased muscle enzyme levels in conjunction with other signs, such as abdominal pain and subcutaneous swelling. Histopathology reveals acute coagulative necrosis of muscle with infarctions. Also, pulmonary hemorrhage and GI infarctions may be present. Even with aggressive corticosteroid therapy and antibiotics, the prognosis is guarded.
Significant rhabdomyolysis with progressive atrophy has been identified in some Quarter Horses after exposure to S. equi.35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree