Fig. 1
BSE epidemic curves and the effect of feed bans for the United Kingdom
3 Feed Bans and Born-After-Reinforced-Ban Cases
Epidemiological analyses of BSE-affected herds identified a protein feed supplement to be the most likely source of infection (Wilesmith et al. 1988), and subsequent recycling of BSE-infected cattle waste in this process may have contributed to the persistence of the disease. Ruminant feed legislation aimed at removing the source of infection from cattle born after 1988 was introduced in 1989–1990 in the UK, with more stringent measures in 1996, and these bans were re-inforced throughout Europe in 2001. The initial UK ban was aimed at prohibiting “cannabalism” by ruminants, end the practice of feeding ruminant-derived meat and bone meal (MBM) to ruminants and so preventing the recycling and amplification of prions in cattle via rendered material. This worked (see Fig. 1) but, geographically, its effect was more noticeable in the south and west than in the north and east of England, the latter area with a higher proportion of pigs to cattle and sheep. This lead to the suspicion that pig and poultry rations (in which ruminant MBM was allowed) were finding their way into ruminant feed and in November 1994 it was prohibited to feed any mammalian protein (with a few exceptions—e.g. blood and milk proteins) to ruminants. After the reporting of a link between BSE and vCJD in 1996, the ban was immediately extended to make illegal the feeding of any mammalian MBM to any farmed livestock, including fish and horses. Ever more stringent feed controls were imposed throughout Europe to limit the spread of BSE within the European Union trade zone and a complete ban on feeding processed animal protein (PAP) of any origin to animals kept, fattened or bred for the production of food was introduced in 2001. Not all countries at risk of BSE worldwide introduced similar exhaustive bans and part of the current dilemma faced by the EU in trying to relax its legislation, in proportion to the diminishing numbers of BSE cases in Europe, is the fear of re-introduction of BSE prions by import of infected material from outside its borders. Feeding pig and poultry processed animal protein to fish is currently under consideration and there is a continuing (unresolved) debate within member states on the use of poultry PAP in pig feed and pig PAP in poultry feed although the risk of the re-amplification of BSE by this usage appears to be vanishingly small (EFSA 2011). Cannabalism, that is feeding PAP of one species to the same species, is widely resisted and there appears to be no European agenda for the re-introduction of feeding ruminant PAP to any species.
At its peak, over 1,000 clinical cases were reported each week in Great Britain in 1993 and the last clinical case was seen by passive surveillance in 2009 (Fig. 1). Active surveillance for disease in fallen stock, in healthy slaughter animals (born after the 1996 feed ban) at the abattoir and in various cohort culls was introduced in 2001 in the UK (and in other member states of the European Union) and, in 2011 when some 500,000 animals were tested, the number of cases detected (all in fallen stock) had dwindled to 7. To date (June 2012), in the UK, only one animal has tested positive for BSE in 2012. Although the feed bans have had a dramatic effect on the epidemic curve, cases of BSE continued to be confirmed in cattle born after the re-inforced European bans of 2000 and feeding of contaminated protein to calves continues to be suspected as the reason for most of these ‘born after the real ban’ (BARB) cases (Ortiz-Pelaez et al. 2012). The biological and biochemical characteristics of BARBs appear similar to those seen in earlier cases in the epidemic, although two of the most recent BARB cases in Britain, including the 2012 case, have had the molecular properties of L-type atypical BSE (see below, Stack et al. in press).
In parallel with the BSE epidemic, natural cases of transmissible spongiform encephalopathies have been also reported for the first time in cattle-related species—greater kudu, eland, nyala and gemsbok, Arabian and scimitar-horned oryx (Cunningham et al. 2004) and in the cat family -puma, cheetahs- and domestic cats. Apart from some cases in the greater kudu, contaminated feed is suspected but difficult to prove because of the absence of detailed feeding records (Cunningham et al. 2004).
4 Minimum Effective Dose of BSE Prions and Specified Risk Material Controls
Experimental oral dosing of cattle with BSE-affected cattle brain homogenates has confirmed that as little as 1 milligram of brain (with ~10–100 mouse ic ID50 units) can induce disease after extended incubation periods of 8–10 years (Arnold et al. 2007, 2009; Wells et al. 2007). Larger doses (up to 100 g) have been used to study oral pathogenesis of BSE in cattle in the UK (Wells et al. 2007) and Germany (Hoffmann et al. 2007). These studies have confirmed by PrP IHC and bioassay the limited, early distribution of prions to parts of the lower alimentary tract (distil ileum, jejunum) and spread via the autonomic nervous system from the gastrointestinal tract to the central nervous system via either the coeliac and mesenteric ganglion complex, splanchnic nerves and the lumbal/caudal thoracic spinal cord or via the vagal nerve. This experimental tissue distribution of infectivity has been used to refine the list of specified risk materials from various age-cohorts of cattle banned for human consumption and has underpinned several assessments of human and animal exposure risk that have defined UK and European policy for control and management of BSE over the years, such as a recent EFSA Opinion on the BSE risk of bovine intestines (EFSA 2010).
5 Atypical Forms of BSE
BSE surveillance testing of cattle for abnormal prion protein in Europe has allowed the identification of two further, distinct types of cattle TSE, termed H- and L-(or BASE) type BSE (Casalone et al. 2004; Biacabe et al. 2007; Jacobs et al. 2007; Polak et al. 2008). Similar cases were also detected outside Europe (Japan and USA) (Hagiwara et al. 2007; Clawson et al. 2008). About 60 atypical BSE cases have been described worldwide (from testing ~50 million healthy animals and fallen stock) although there is no statutory requirement to distinguish typical and atypical types of BSE in reporting and this figure is derived from research literature.
In France, a retrospective study of all the TSE-positive cattle identified through the compulsory EU surveillance programme between 2001 and 2007 was recently published (Biacabe et al. 2008). This study indicated that all BSE H and L cases detected by rapid tests were observed in animals over 8 years old in either the “at risk” (9) or “healthy slaughtered” surveillance target group (4). In this study, the reported frequency of H and L type TSE was respectively 1.9 and 1.7 cases per million of over 8-year-old tested animals. All EU atypical cases were born before the extended or real feed ban that came into law in January 2001. Hence, as with classical BSE, exposure of these animals to feed contaminated with low titres of TSE cannot be excluded. However, the distribution of H-and L-type cases in France by year of birth differs markedly from that for classical BSE and could be interpreted to indicate that both forms of atypical BSE are sporadic diseases which arise spontaneously. Indeed, a case of H-type BSE in the USA has been associated with a inheritable, bovine E211 K mutation (that is the amino-acid lysine replacing glutamic acid at codon 211) in the wild-type prion protein amino-acid sequence and shown to be transmissible by intra-cranial inoculation to a calf carrying the same mutated allele with a post-inoculation survival time of 301 days (Greenlee et al. 2012); however, DNA sequencing of the PrP open reading frame of other atypical cases has not identified this or any other coding region mutation.
H- and L-(or BASE) type BSE have been transmitted by intra-cerebral challenge to inbred mice and Tg mice expressing bovine and ovine PrP. L-type BSE has also been transmitted to transgenic mice expressing alleles of the human prion protein (Buschmann et al. 2006; Beringue et al. 2008; Kong et al. 2008). Transmission and serial passage in inbred mice and Tg VRQ mice have been interpreted to indicate that, after interspecies passage, BASE could generate classical BSE (Beringue et al. 2007; Capobianco et al. 2007). However, it should be noted that L-BSE—classical BSE phenotypic convergence has not been observed in other Tg mice, including mice expressing the ARQ allele of sheep PrP (Buschmann et al. 2006; Beringue et al. 2007). Prions with the properties of a classical BSE strain also emerged during serial passage of H-type BSE in wild-type mice (Baron et al. 2011). These phenomena need to be confirmed in an independent set of experiments but do raise the issue of a possible classical BSE re-emergence originating from atypical BSE cases; mutation rates of 1 in 100,000 have been calculated for conversion (or re-version) of one prion strain to another during passage in cell culture (Oelschlegel et al. 2012; Weissmann 2012) but a similar estimate of the probability of one type of prion changing to another (for example, from a non-zoonotic scrapie to zoonotic BSE phenotype) has not been determined for either experimental or natural transmission routes in animals.
The sensitivity and specificity of the TSE rapid screening tests are known for classical BSE but not for H- or L-type BSE. These tests use brainstem as the target tissue because this is where pathological lesions and PrPres are first detected in the CNS of cattle (Hope et al. 1988; Wells et al. 1998). Unlike classical BSE, little is known about the pathogenesis of atypical BSE and the brainstem may not be the optimal target site for the detection of H- and L- type BSE (Casalone et al. 2004). Consequently the BSE H- and L- type prevalence of 1–2 per million may be an under-estimation. H-type and L-type BSE have been transmitted to cattle (Lombardi et al. 2008; Fukuda et al. 2009) and the molecular and pathological characteristics of each type has been maintained and differ from those of classical BSE (Balkema-Buschmann et al. 2011; Okada et al. 2011a, b; Konold et al. 2012). Some data are now emerging on the distribution of the infectivity in peripheral tissues of cattle with atypical BSE (Iwamaru et al. 2010; Okada et al. 2011a, b; Suardi et al. 2012) and, although limited compared to the distribution of prions in small ruminants, various skeletal muscles in L-type (BASE) cattle were found to contain infectivity (detected by bioassay in Tgbov mice) and PrP-immuno-reactive deposits within individual muscle fibres (Suardi et al. 2012).
6 Small Ruminant BSE
Foster and colleagues showed cattle BSE could be transmitted to ARQ/ARQ sheep and goats by feeding and intra-cerebral inoculation (Foster et al. 1993) and several subsequent studies have documented that there is wide-spread dissemination of BSE prions in ARQ/ARQ sheep similar to the pathogenesis of natural cases of classical scrapie (van Keulen et al 2000). The biological and biochemical characteristics of “BSE in small ruminants” are sufficiently distinct to allow their discrimination in “blinded” tests although there have been concerns “mixed” infections might pass as “scrapie”. Historically, small ruminants were known to have been fed the same type of protein supplements implicated as the source of BSE in cattle and fear of a second wave of vCJD due to infection from sheep and goat products stimulated intensive surveillance in the EU of TSEs in sheep and goats and the application of laboratory tests aimed at a diagnosis of “NOT BSE” or “BSE NOT Excluded”; the final confirmation of “BSE in small ruminant” requires the application of bioassay in the same panels of inbred mice used to characterise vCJD and BSE (Bruce et al. 1997). By these stringent criteria, only two cases of BSE in small ruminants(SRs), both in goats, have been confirmed (Eloit et al. 2005; Jeffrey et al. 2006) and current estimates of the likely prevalence of BSE in SRs in Europe is very low.
7 Variant Creutzfeldt-Jakob Disease
In April 1996, Will and colleagues (Will et al. 1996) reported a novel variant of CJD (vCJD). The initial, and subsequent, focus of vCJD in Great Britain and its molecular (Collinge et al. 1996) and transmission (Lasmezas et al. 1996; Bruce et al. 1997) similarities to BSE immediately implicated the cattle disease as the source of vCJD infection (Fig. 2) and beef and cattle by-products were put under further restrictions to limit the spread of disease. Nevertheless, an estimated three million infected cattle may have entered the human food chain (Ghani et al. 2000) and the impact and cost of preventing a secondary, human-to-human wave of infection are still being felt in the UK (Garske and Ghani 2010).
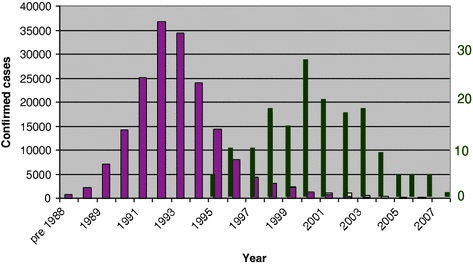

Fig. 2
BSE and vCJD in the UK: temporal link. Left-hand scale, thick bars, BSE cattle; right-hand scale, thin bars, vCJD cases
To date (June 2012), there have been 176 primary cases, and three secondary cases related to transfusion of blood products, in the UK, 26 cases in France, 5 in Spain and 16 in the rest of Europe; other cases have been reported in the United States, Canada, Saudi Arabia, Taiwan and Japan (www.cjd.ed.ac.uk/vcjdworld). Polymorphisms and mutations in the human prion protein gene are known to affect the survival and clinico-pathological phenotype of human TSEs and the vCJD is clearly associated with the dimorphism at codon 129 (Methionine or Valine): the percentage of this dimorphism in the normal Caucasian population is 39 MM, 50 MV and 11 % VV; in cases of sporadic CJD, the proportions are 65 MM, 17 MV and 18 % VV. All clinical cases of vCJD so far diagnosed are MM homozygous although pre/sub-clinical disease has been inferred from PrPCJD detection in the spleen of a blood transfusion recipient who was an M/V heterozygote (Peden et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


