Radiographic technique for the spine115
5.1. RADIOGRAPHIC TECHNIQUE FOR THE SPINE
5.2. VARIATIONS IN VERTEBRAL NUMBER
5.3. VARIATIONS IN VERTEBRAL SIZE AND SHAPE: CONGENITAL OR DEVELOPMENTAL
5.4. VARIATIONS IN VERTEBRAL SIZE AND SHAPE: ACQUIRED
Increased vertebral size
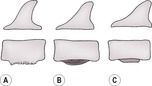
Figure 5.7
Decreased vertebral size
Altered vertebral shape
Vertebral canal changes
5.5. VARIATIONS IN VERTEBRAL ALIGNMENT
5.6. DIFFUSE CHANGES IN VERTEBRAL OPACITY
Generalized decrease in radiopacity of the vertebrae (see also 1.16, Osteopenia)
Generalized increase in radiopacity of the vertebrae (see also 1.13, Increased radiopacity within bone)
5.7. LOCALIZED CHANGES IN VERTEBRAL OPACITY
Localized decrease in radiopacity of one or more vertebrae (see also 1.18, Osteolytic lesions and 1.19, Expansile osteolytic lesions)
Localized increase in radiopacity of one or more vertebrae
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Spine
5.2 Variations in vertebral number116
5.3 Variations in vertebral size and shape: congenital or developmental117
5.4 Variations in vertebral size and shape: acquired119
5.5 Variations in vertebral alignment122
5.6 Diffuse changes in vertebral opacity124
5.7 Localized changes in vertebral opacity124
5.8 Abnormalities of the intervertebral disc space126
5.9 Irregularity of the vertebral endplates127
5.10 Abnormalities of the intervertebral foramen128
5.11 Abnormalities of the articular facets129
5.12 Lesions in the paravertebral soft tissues130
5.13 Ultrasonography of paravertebral soft tissues130
5.14 Spinal contrast studies: technique and normal appearance131
5.15 Technical errors during myelography133
5.16 Extradural spinal cord compression on myelography134
5.17 Intradural–extramedullary spinal cord compression on myelography137
5.18 Intramedullary spinal cord enlargement on myelography138
5.19 Miscellaneous myelographic findings139
5.20 Changes on plain radiographs that are unlikely to be significant140
5.21 Neurological deficits involving the spinal cord or proximal nerve roots with normal plain radiographs and myelogram140
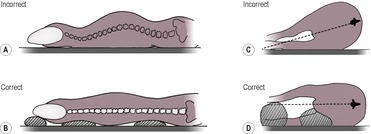
Optimal radiographs are obtained with the patient under sedation or general anaesthesia to minimize motion blur and allow accurate positioning.
True lateral and ventrodorsal (VD) positioning should be ensured by the use of positioning aids (Fig. 5.1A–D). At the lumbosacral junction, a VD radiograph obtained with the area flexed may also be helpful. In the cervical spine, oblique views increase the visibility of the intervertebral foramina, the dens (odontoid peg) and the occipital condyles. Horizontal VD views are desirable when severe instability or spinal fractures are suspected to avoid additional injury on manipulation of the patient. Detail intensifying screens are preferred, and a grid should be used if the tissue thickness is greater than 10 cm. Close collimation will also improve image definition by reducing the production of scattered radiation. If neurological deficits are present or if disc disease is suspected, the primary beam must be centred at the level of the suspected lesion.
Myelography used to be the most commonly used contrast medium technique for the evaluation of the spinal cord or cauda equina but in many establishments is being replaced by cross-sectional imaging techniques. Myelography is discussed further in Section 5.14. Epidurography, discography and lumbar sinus venography are additional techniques that are sometimes still used to evaluate the cauda equina but that are also becoming obsolete. Linear tomography is useful in the thoracic and lumbosacral regions to eliminate superimposition of the ribs and ilial wings, respectively. Cross-sectional images can be obtained by means of CT and MRI; CT provides better definition of bone and joint abnormalities, whereas MRI provides high soft tissue contrast and is ideal for cases with no survey film abnormalities, such as spinal tumours, early infection and ligamentous pathology. CT myelography can be performed to increase the information given about changes in cord size. Scintigraphy is occasionally used to identify the location of inflammatory or neoplastic processes but gives little anatomical detail.
Optimal interpretation of spinal radiographs requires a systematic evaluation that involves assessing radiographic quality and technique, extravertebral soft tissue structures, osseous vertebral structures, disc spaces and adjacent vertebral endplates, intervertebral foramina and articular facets. Each vertebra, disc space and intervertebral foramen should be compared with those adjacent to them. Disc spaces normally appear narrower towards the periphery of the film due to divergence of the primary X-ray beam.
Vertebral physes should be closed by 38 weeks, with cranial physes closing before caudal physes. The apex and body of the dens (odontoid peg) have separate ossification centres and are completely ossified by 25 weeks.
The normal vertebral formula in the dog and cat is 7 cervical, 13 thoracic, 7 lumbar, 3 sacral and a variable number of caudal vertebrae. Numerical alterations may be genuine or may be accompanied by other congenital vertebral abnormalities, which may result in apparent vertebral number alterations (transitional vertebrae – see 5.3.2).
1. Six or eight lumbar vertebrae (especially Dachshund).
2. Four sacral vertebrae – vestigial disc spaces may be visible.
3. Twelve thoracic vertebrae.
a. Twelve genuine thoracic vertebrae and seven lumbar vertebrae.
b. T13 lacks ribs, giving the appearance of 12 thoracic and 8 lumbar vertebrae.
4. Fourteen thoracic vertebrae – usually due to the presence of rib-like structures on L1 rather than a genuine increase in number.
5. Inherited short tail (brachyury) in the Manx cat (‘stumpies’) and certain dog breeds, including the Pembroke Welsh Corgi, Brittany Spaniel, Bouvier des Flandres, Swedish Vallhund and Polish Lowland Sheepdog. In the Manx cat, other spinal deformities are often associated.
6. Inherited taillessness (anury); especially the Manx cat (‘rumpies’), and associated with other spinal deformities (see 5.3.20).
7. Perosomus elumbis – rare neuroskeletal congenital defect in which there is agenesis of the lumbosacral spinal cord and vertebrae, with associated pelvic limb deformities; mainly cattle but has been reported in dogs.
More than one abnormality may be present.
1. Normal variants.
a. The neural arch of C1 is often short in toy breeds of dog.
b. C7 and L7 may be shorter than the adjacent vertebrae.
c. The ventral margins of L3 and L4 vertebral bodies are often poorly defined due to bony roughening at the origins of the diaphragmatic crura.
2. Transitional vertebrae – these are vertebrae that have anatomical features of two adjacent regions. They are commonly seen and may accompany numerical abnormalities, but other than those at the lumbosacral junction they are not usually clinically significant. The transitional segment may show unilateral or bilateral changes.
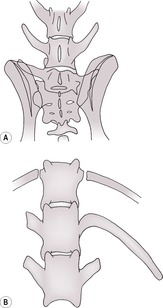
a. Sacralization of the last lumbar vertebra (Fig. 5.2A) – one or both transverse processes fuse to the wing of the sacrum and may also articulate with the ilium. Asymmetrical and symmetrical transitional vertebrae have roughly equal incidence. This may predispose to lumbosacral instability and disc degeneration with secondary cauda equina syndrome. If rotational malalignment is present, it may also predispose to unilateral hip dysplasia and result in an inability to obtain pelvic symmetry during positioning for hip dysplasia radiographs; this is more likely to occur with unilateral sacralization. Common in the German Shepherd dog but also in the Dobermann, Rhodesian Ridgeback, Greater Swiss Mountain dog and Brittany Spaniel.
b. Lumbarization of S1 vertebra, which fails to fuse to the rest of the sacrum (Fig. 5.2A); appears similar to the above radiographically and may be differentiated only by counting the number of normal adjacent vertebral segments.
c. Partial or complete fusion of S3 to Cd1; also common in cats, particularly Burmese. Pseudoarticulation of the transverse processes may be present. Often seen with (b) in an attempt to restore three sacral segments.
d. Transitional T13 vertebra (Fig. 5.2B) – a rib develops into a transverse process; a vestigial rib may be seen as a mineralized line in the soft tissues.
e. Transitional L1 vertebra – a transverse process elongates and develops into a rib-like structure; also common in cats.
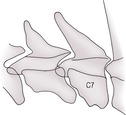
f. Transitional C7 vertebra – a transverse process elongates and develops into a rib-like structure.
g. Occipitalization of the atlas.
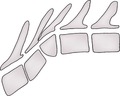
3. Hemivertebrae (Fig. 5.3) – malformation of the vertebral body; a common abnormality in the thoracic and tail regions, particularly in screw-tailed breeds and the German Short-haired Pointer. Rare in cats. Multiple vertebrae are often affected. Clinical signs (neurological deficits due to spinal cord compression) are uncommon and usually occur in the first year of life during the growth phase.
a. Dorsal hemivertebra – ventral half did not develop, producing kyphosis.
b. Lateral hemivertebra – left or right half did not develop, producing scoliosis.
c. Ventral hemivertebra – dorsal half did not develop, producing lordosis.
4. Block or fused vertebrae – usually only two and rarely three segments are fused – reduced or absent disc space with vertebrae of normal length. The degree of fusion varies. The increased stress on adjacent disc spaces predisposes to subsequent disc herniation. Differential diagnoses are old trauma, healed discospondylitis.
a. Lumbar region.
b. Cervical region.
5. Butterfly vertebrae (Fig. 5.4) – particularly brachycephalic breeds of dog; rare in cats. Unlikely to cause clinical signs. Seen on the VD view, particularly in the caudal thoracic and caudal lumbar regions as a cleft of the cranial and caudal vertebral endplates due to partial sagittal cleavage of the vertebral body.
6. Incomplete fusion of sacral segments.
8. Cervical spondylopathy (wobbler syndrome; see 5.4.20 and Fig. 5.5) – especially Dobermann. Malformed caudal cervical vertebrae, often with a plough-share appearance of the centrum and wedge-shaped disc spaces; the neural arch may also be angled cranioventrally, and both of these result in vertebral canal stenosis. Spinal cord compression may be worsened on flexion of the neck due to vertebral instability, creating a dynamic lesion. However, neurological signs are seen in young animals only when stenosis is severe. See 5.4.14 for description of acquired changes.
9. Narrowed vertebral canal (spinal stenosis) – needs myelography or MRI to demonstrate the degree of cord compression.
a. Secondary to hemivertebrae or block vertebrae.
b. Cervical spondylopathy (wobbler syndrome; Fig. 5.5).
c. Thoracic stenosis.
– T3–6 usually with no cord compression – Dobermann.
– Individual thoracic vertebrae – Bulldog.
10. Sacral osteochondrosis – clinical signs not usually apparent until skeletally mature (see 5.4.16 and Fig. 5.8).
11. Congenital metabolic disease affecting vertebrae at a young age.
a. Pituitary dwarfism – especially German Shepherd dog; proportionate dwarfism ± epiphyseal dysgenesis.
b. Congenital hypothyroidism – especially Boxer; disproportionate dwarfism with epiphyseal dysgenesis leading in the spine to delayed vertebral endplate ossification and growth plate closure; endplates may show characteristic ventral spikes. Pathological fracture through an unfused growth plate has been reported. Long bone changes also occur (see 1.22.9).
12. Fused dorsal spinous processes.
13. Spina bifida – results in a split or absent dorsal spinous process or absent lamina, most common in the caudal lumbar region; especially the Bulldog and rare in cats. A widened vertebral canal may be seen on the lateral view. May be accompanied by spinal dysraphism, a defective closure of the neural tube. Myelography is required to assess soft tissue changes.
a. Spina bifida occulta – normal spinal cord and intact skin. Common in short-tailed breeds.
b. Meningocele – herniated meninges, skin intact.
c. Myelomeningocele – herniated spinal cord and meninges, skin intact.
d. Spina bifida manifesta – herniated spinal cord and meninges exposed to the exterior.
e. Spina bifida cystica – herniated spinal cord and meninges elevated above the skin.
14. Dermoid (pilonidal) sinus – occasionally associated with defects in the dorsal spinous process or neural arch, if the sinus tract extends to the dura (see 5.7.4).
15. Occipitoatlantoaxial malformations – combinations of abnormalities including occipital dysplasia, fusion of the occiput to the atlas, short atlas neural arch, deformities of the dens and atlantoaxial subluxation; clinical signs depend on the degree of resulting cord compression, and some may be clinically silent.
16. Other occasional complex vertebral anomalies, especially in the cervical area.
17. Articular facet aplasia.
a. Cervical.
b. Thoracolumbar, often with hyperplasia of the adjacent facet.
18. Idiopathic multifocal osteopathy of the Scottish Terrier (see 1.18.7); absence of parts of vertebrae may occur, particularly dorsally in the cervical spine.
19. Perocormus – severe shortening of the vertebral column.
20. Cats – sacrococcygeal (sacrocaudal) dysgenesis; varies from spina bifida to complete sacrococcygeal agenesis. Especially in Manx cats, in which it may be accompanied by other anomalies such as shortened cervical vertebrae, butterfly vertebrae, fusion of lumbar vertebrae and meningo(myelo)cele.
21. Cats – mucopolysaccharidosis – congenital lysosomal storage disease, although signs may not manifest until later in life (see 5.4.9).
For articular facet variations, see 5.11.
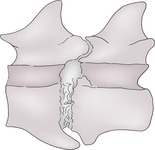
1. Spondylosis deformans – varying sizes of ventral and lateral bony spurs that may bridge the disc space (Fig. 5.6). Usually clinically insignificant unless so extensive as to result in nerve root involvement; at the lumbosacral junction it may be associated with other pathology and cauda equina syndrome.
a. Initiated by degeneration of annulus fibrosus – an incidental finding that may start as young as 2 years, is very common and increases in incidence with age; less common and generally milder in cats.
c. Syndesmitis ossificans – extensive ossification of the ventral longitudinal ligament – young Boxers.
3. Neoplasia.
a. Benign neoplasia or disorder of skeletal development.
– Single osteochondroma or multiple cartilaginous exostoses, often producing expansile lesions of the dorsal spinous processes in young animals. Growth ceases at skeletal maturity in dogs, but lesions may continue to grow after the active growth phase in cats (see 1.15.2). Malignant transformation to osteosarcoma or chondrosarcoma has been reported.
b. Malignant neoplasia.
– Osteosarcoma.
– Other primary or metastatic tumours.
c. Hindquarter soft tissue tumours resulting in ventral periosteal reaction on caudal lumbar and sacral vertebrae (Fig. 5.7; differential diagnosis is spondylitis – see below, but neoplastic changes are usually more caudal). The new bone is not necessarily neoplastic but is a reaction to local malignancy.
– Prostatic tumour – the most common cause of such new bone.
– Bladder or urethral tumour.
– Perianal tumour.
– Mammary tumour.
New bone on the ventral margins of vertebral bodies due to local neoplasia (usually L5–7) or spondylitis (usually L1–3). The new bone may be brushlike (A), lamellar (B) or solid (C). See 1.6 for further description of periosteal reactions.
4. Spondylitis (Fig. 5.7) – usually characterized by vertebral body periosteal reactions, particularly ventrally, and may progress to osteomyelitis of the vertebral body. Conversely, osteomyelitis may also originate haematogenously within the vertebra and extend peripherally. Note that the ventral margins of L3 and L4 are often poorly defined due to bony roughening at the origins of the diaphragmatic crura, and this should not be mistaken for spondylitis.
a. Bacterial.
– Migrating plant material – especially ventral to L1–3 (T13–L4). Medium and large-sized dogs are thought to aspirate vegetation such as grass awns, which migrate through the lung and diaphragm to the origin of the crura at L3 and L4; an alternative proposed route is via penetration of the gastrointestinal tract. May be associated with paraspinal abscessation, and such changes may be visible with ultrasonography (see 5.13).
– Other foreign bodies.
– Haematogenous infection.
– Bite wounds.
– Iatrogenic due to surgical complications.
b. Parasitic.
– Spirocerca lupi* – spondylitis of mid-thoracic vertebrae (T5–11); with caudal mediastinal (oesophageal) mass (see 8.19.5).
c. Fungal.
– Actinomycosis*.
– Coccidiodomycosis*.
– Aspergillosis,* especially German Shepherd dogs.
5. Disseminated (diffuse) idiopathic skeletal hyperostosis – similar to the disease of the same name in humans and seen in large and giant dogs; the main changes are in the spine, with extensive, flowing new bone formation along the ventral and lateral margins of the vertebral bodies and at sites of ligamentous attachments; also extremital periarticular new bone and enthesiophyte formation. Aetiology unknown but appears to represent an exaggerated response to stimuli that would normally induce little, if any, new bone formation and may be considered to be an ossifying diathesis.
6. Baastrup’s disease – bony proliferation between dorsal spinous processes. Larger dog breeds, especially Boxer.
7. Aneurysmal bone cyst (see 1.19.3).
8. Cats – hypervitaminosis A; extensive new bone formation on cervical and cranial thoracic vertebrae and rarely further caudally. Mainly ventrally, mimicking severe spondylosis, but may also involve the sides and dorsum of the vertebrae. Long bone joints may also be affected at sites of soft tissue attachments, especially the elbow and stifle. Ankylosis of the spine and affected limb joints may occur. Usually 2- to 9-year-old cats on raw liver diets; differential diagnosis is mucopolysaccharidosis.
9. Mucopolysaccharidosis; lysosomal storage diseases causing new bone on the vertebrae, which may lead to spinal fusion, and endplate dysplasia; also dwarfism, facial deformity, pectus excavatum, hip dysplasia and epiphyseal dysplasia leading to osteoarthrosis. More common in cats, especially those with Siamese ancestry; rare in the dog; differential diagnosis is hypervitaminosis A. Mucopolysaccharidosis type VII has been reported to cause shortened vertebral bodies and irregular vertebral epiphyses as well as disproportionate dwarfism in a German Shepherd dog.
10. Fractures – may result in shortened vertebra due to compression.
11. Discospondylitis – osteolysis of vertebral endplates eventually results in a shortened vertebral body, with secondary spondylosis deformans and even fusion in the later stages (see 5.9.1 and Fig. 5.10).
12. Indented vertebral endplates.
a. Intravertebral disc herniation (Schmorl’s node) – particularly L7 and/or S1; medium and large breeds, especially German Shepherd dog.
b. Nutritional secondary hyperparathyroidism (juvenile osteoporosis) – the central part of the endplate is indented by the nucleus pulposus while the bones are soft and then remains for life.
13. Mucopolysaccharidosis; the vertebrae may be shortened or misshapen because dwarfism and epiphyseal dysplasia may be a feature (see 5.4.9).
14. Cervical spondylopathy (wobbler syndrome) – congenitally malformed ± unstable cervical vertebrae that may be accompanied at a later age by acquired changes such as remodelling of the centrum, endplate sclerosis, spondylosis and secondary disc herniation. Especially Dobermann (see 5.3.8 and Fig. 5.5). Most cases present in middle age due to secondary disc herniation, although in cases of severe deformity neurological signs are evident at a younger age. If MRI is not available, myelography is required to demonstrate acquired soft tissue changes and to quantify the degree of cord compression, which may be due to primary bony deformity or to secondary disc herniation, facet arthrosis or ligamentum flavum hypertrophy or redundancy (see also 3., 4. and 5., 5.16.7 and 5.16.8).
15. Fractures – the vertebrae may be misshapen due to malunion or asymmetric compression.
16. Sacral osteochondrosis (Fig. 5.8) – remodelling (angulation, lipping, bone defect, subchondral sclerosis) of the craniodorsal aspect of S1 and rarely the caudodorsal aspect of L7 ± an osteochondral fragment; mainly young male German Shepherd dogs but also Boxers and Rottweilers. On the VD view, seen to be paramedian or bilateral rather than midline. Myelography may show deviation or compression of the dural sac. Although a developmental disease, clinical signs are not usually seen until > 18 months of age.
17. Neoplasia (see 5.4.3).
18. Mucopolysaccharidosis (see 5.4.9).
19. Widened vertebral canal.
a. Normal at the level of the cervical (C5–T2) and lumbar (L2–5) intumescentia.
b. Enlarged spinal cord due to chronic pathology.
– Tumour (e.g. slow-growing astrocytoma, ependymoma, meningioma).
– Syringohydromyelia, especially at the level of C2; common in the Cavalier King Charles Spaniel.
c. Spinal arachnoid pseudocyst.
20. Narrowed vertebral canal.
a. Cervical spondylopathy (wobbler syndrome).
– Lateral view; dorsoventral narrowing at the cranial end of the affected vertebra.
– VD view; medially deviating pedicles of the caudal vertebral canal, particularly in the Great Dane and Boerboel.
b. Expansile or healing lesions of adjacent bone.
c. Lumbosacral stenosis.
d. Calcium phosphate deposition disease in Great Dane pups – dorsal displacement of C7 accompanied by deformation of the articular facets.
The floor of the vertebral canal of adjacent vertebrae should form a continuous straight to gently curved line. Malalignment may be constant and visible on survey radiographs, or intermittent and require radiographs made while the region is flexed or extended (stress radiography) to demonstrate instability.
1. Scoliosis (lateral curvature).
a. Muscular spasm.
c. Pathological fracture.
d. Spinal cord abnormalities leading to functional scoliosis.
– Dandy–Walker syndrome.
– Spinal dysraphism – Weimaraner.
– Syringohydromyelia; especially Cavalier King Charles Spaniel.
2. Lordosis (ventral curvature).
a. Normal conformational variant.
b. Muscular spasm.
d. Loss of fibrotic vertebral support – old and heavy dogs.
e. Pathological fracture.
f. Nutritional secondary hyperparathyroidism (juvenile osteoporosis), especially caudal lumbar spine (see 1.16.4).
3. Kyphosis (dorsal curvature).
a. Normal conformational variant.
b. Muscular spasm.
e. Discospondylitis.
f. Pathological fracture.
g. Nutritional secondary hyperparathyroidism (juvenile osteoporosis) (see 1.16.4).
4. Trauma: a three-compartment model may be used to assess the stability of spinal fractures. Each vertebra is divided into a dorsal compartment (articular processes, spinous processes, laminae, pedicles, supporting soft tissues), a middle compartment (dorsal longitudinal ligament, dorsal part of annulus fibrosus, dorsal aspect of vertebral body) and a ventral compartment (remainder of vertebral body and annulus fibrosus, nucleus pulposus, ventral longitudinal ligament). If two of the three compartments are damaged, the fracture is considered to be unstable. CT is more reliable than radiography for determination of the extent of spinal fractures. Remember that bony displacement at the time of injury may have been greater than is recognized radiographically, having been reduced again by muscle spasm. Subsequent callus formation may cause worsening of neurological deficits some time after the injury. Small chip fractures may also be seen due to avulsion of soft tissue attachments.
a. Fracture.
b. Subluxation.
c. Luxation.
d. Salter–Harris type I or II fracture through vertebral physis in immature animal, with displacement of the epiphysis.
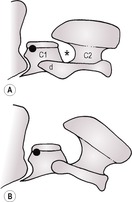
5. Atlantoaxial subluxation (Fig. 5.9) – abnormal alignment of C2 (axis) relative to C1 (atlas), which results in widening of the intervertebral space between the neural arch of C1 and the cranioventral aspect of the spine of C2, exacerbated by mild neck flexion (note that subtle widening of this space occurs in normal subjects on flexion). Dorsal subluxation of C2 may also occur. Marked flexion must be avoided, as it may cause spinal cord damage in the presence of instability. Usually due to defects of the dens (odontoid peg), evaluation of which is best achieved on oblique or VD views of the neck. The rostrocaudal open mouth view has been described for evaluation of the dens but in the presence of instability is likely to cause spinal cord damage. Clinical signs of neck pain and varying degrees of motor dysfunction result.
a. Congenital atlantoaxial instability – younger miniature and toy breeds (especially Yorkshire Terrier, Toy Poodle, Chihuahua, Pomeranian and Maltese), rarely large-breed dogs. May present clinically at a later age due to superimposed minor trauma.
– Aplastic dens.
– Hypoplastic dens.
– Non-fusion of the dens to C2 (fusion normally completed at 6 months).
– Absence of the dens ligaments.
– Cats – dens agenesis resulting from mucopolysaccharidosis (see 5.4.9).
b. Acquired.
– Fracture of the dens or cranial part of C2.
– Rupture of the transverse ligament of the atlas, which normally holds the dens against the floor of that vertebra.
6. Cervical spondylopathy (wobbler syndrome) (see 5.3.8 and Fig. 5.5) – mainly Dobermann.
a. Static – malformed caudal cervical vertebrae with craniodorsal subluxation (tipping) of one or more vertebrae and a dorsoventrally narrowed cranial vertebral canal opening. Often accompanied by wedge-shaped or narrowed disc spaces and spondylosis.
b. Dynamic – malalignment worsens with ventroflexion of the neck.
7. Lumbosacral instability – step formation between the last lumbar and first sacral vertebrae. May be seen only on stress radiography of the region. Common in the German Shepherd dog, in which transitional lumbosacral vertebrae may predispose to instability; may progress to degenerative lumbosacral stenosis and cauda equina syndrome (see 5.16.6 and Fig. 5.17).
8. Calcium phosphate deposition disease in Great Dane pups – dorsal displacement of C7 accompanied by deformation of the articular facets.
1. Artefactual generalized decrease in vertebral radiopacity.
a. Overexposure.
b. Long-scale exposure techniques (high kV, low mAs).
c. Obese or large patients creating large amounts of scattered radiation, especially if a grid was not used or with inadequate collimation.
d. Overdevelopment.
e. Fogging of the film (numerous causes).
2. Metabolic bone disease.
a. Secondary hyperparathyroidism.
b. Primary hyperparathyroidism.
c. Corticosteroid excess.
– Hyperadrenocorticism – Cushing’s disease.
– Iatrogenic – long-term corticosteroid administration.
d. Hyperthyroidism.
e. Diabetes mellitus.
f. Congenital hypothyroidism – especially Boxer; delayed closure of vertebral physes and dysgenesis of endplates.
g. Pseudohyperparathyroidism; hypercalcaemia of malignancy.
h. Osteogenesis imperfecta – long bone changes usually predominate.
3. Senile osteoporosis – especially aged cats.
4. Neoplastic.
a. Plasma cell myeloma (multiple myeloma) – genuine osteopenia as well as multiple osteolytic lesions (see 1.18.2).
5. Cats – hypervitaminosis A (raw liver diets); although proliferative bony changes predominate and mask the osteopenia (see 5.4.8).
6. Cats – mucopolysaccharidosis; likewise (see 5.4.9).
7. Artefactual generalized increase in vertebral radiopacity.
a. Underexposure.
b. Underdevelopment.
8. Osteopetrosis–osteosclerosis complex – hereditary in the Basenji.
9. Fluorosis.
10. Cats – feline leukaemia virus-associated medullary sclerosis.
1. Artefactual localized decrease in vertebral radiopacity.
a. Superimposed skin defect, bowel or lung air on rotated lateral or VD views.
b. Superimposed subcutaneous gas.
2. Decreased radiopacity of the vertebral endplate.
b. Neoplasia (see below).
c. Intravertebral disc herniation (Schmorl’s node) – particularly at L7 and/or S1; medium and large breeds, especially German Shepherd dog.
3. Irregular or discrete radiolucencies – single or multiple radiolucent areas involving single or multiple vertebrae and that may be accompanied by bone production. Pathological fracture may occur.
a. Primary tumour – usually only one vertebra involved; less common than neoplasia in the appendicular skeleton; often primarily osteolytic.
– Osteosarcoma.
– Fibrosarcoma.
– Chondrosarcoma.
– Haemangiosarcoma.
– Solitary plasma cell myeloma – characteristic punched-out appearance with little surrounding reaction.
– Others.
b. Metastatic or multifocal tumours – may involve multiple vertebrae or other bones; often primarily osteolytic.
– Multiple myeloma (plasma cell myeloma).
– Lymphoma.
– Histiocytic sarcoma (malignant histiocytosis) – especially Rottweiler, Bernese Mountain dog, Golden Retriever, Flat-coated Retriever.
– Metastases from, for example, prostatic, mammary, thyroid carcinomas; osteosarcoma.
c. Infiltrative soft tissue tumours – may involve more than one vertebra, or a vertebra and adjacent bone (rib, pelvis); an adjacent soft tissue mass may be evident.
– Haemangiosarcoma.
– Lymphoma.
– Fibrosarcoma.
– Rhabdomyosarcoma.
– Others.
d. Benign tumours – osteochondroma or multiple cartilaginous exostoses may produce expansile osteolytic lesions (see 1.15.2).
e. Severe spondylitis – osteolysis and pathological fracture are reported with systemic aspergillosis*.
f. Aneurysmal bone cyst.
g. Dermoid or epidermoid cyst.
h. Post surgery (e.g. hemilaminectomy bone defect).
4. Linear radiolucencies.
a. Fractures.
b. Widened vertebral physis.
– Salter–Harris growth plate fractures in skeletally immature animals.
– Vertebral physitis – haematogenous infection in young dogs, affecting mainly caudal lumbar physes, where blood flow is sluggish; may be associated with portosystemic shunts.
c. Dermoid (pilonidal) sinus extending from the dorsal midline usually to cervical or cranial thoracic vertebrae; especially in young Rhodesian Ridgebacks, in which it shows autosomal recessive inheritance; sporadic in other breeds. Variable depth, in some cases extending to the dural sac either through the interarcuate space or via a neural arch defect, the latter appearing as a linear radiolucency. Clinical signs occur if the sinus involves the meninges and/or becomes infected. Sinography using non-ionic contrast medium may be used to detect the depth of the sinus.
5. Artefactual localized increase in vertebral radiopacity.
a. Superimposed structures (e.g. extruded, mineralized disc material superimposed over neural arch).
b. Underexposure of thicker areas of tissue.
6. Inflammation or osteomyelitis – it may not be possible to differentiate periosteal new bone superimposed over the vertebra from increased density of the bone itself (sclerosis).
a. Spondylosis.
b. Discospondylitis.
c. Spondylitis.
7. Neoplasia – although osteolysis usually predominates, areas of increased radiopacity may also be visible and some tumours are genuinely sclerotic.
a. Osteogenic osteosarcoma.
b. Chondrosarcoma.
c. Osteochondroma or multiple cartilaginous exostoses (see 1.15.2).