Author
Year
Model
Monchik and Russell [1]
1971
Heterotopic SBT with systemic venous drainage
Kort [4]
1973
Orthotopic SBT with portal drainage
Schraut [5]
1983
Heterotopic SBT with portal drainage
Deltz and Thiede [6]
1985
Two-stage SBT (heterotopic- orthotopic)
Wallander [7]
1988
SBT with using “cuff technique” for vascular connection
Szymula von Richter [8]
1996
SBT with reconstruction of lymphatic vessel
13.2.1 Heterotopic SBT
In this model, the recipient’s own small bowel is left in the abdomen and the graft is not connected into the intestinal tract of recipient. The graft venous outflow is reestablished using the vena cava caudalis (Fig. 13.1). The main advantage of this model is relatively easy technical implementation contrary to other models and the possibility to easily perform enteral biopsy of the graft. Even if the heterotopic model of SBT does not imitate the physiological conditions as well as orthotopic transplantation , it is suitable for immunological study and the study of ischemic reperfusion injury.
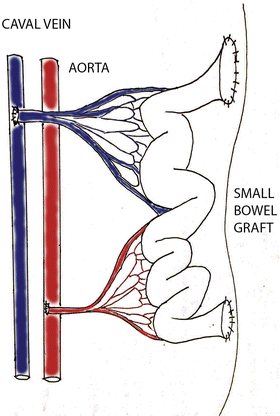

Fig. 13.1
Heterotopic SBT with two entero stoma (sketched by Dr. Petr Wohl)
13.2.2 Orthotopic SBT with Systemic Venous Drainage
Unlike heterotopic transplantation , in this model the small bowel of the recipient is removed and replaced with a donor’s graft. Venous drainage is re-established with porto-caval anastomosis. Compared to heterotopic transplantation, this model is associated with increased mortality though a skilled surgeon could achieve more than 85 % of animal survival [9] (Fig. 13.2). The main advantage of this model is that the entire graft is exposed to normal intraluminal environment of recipient, which consists of nutrition, gastrointestinal juice and germs and thriving of recipient absolutely depends on functional integrity of the bowel graft. Orthotopic SBT is suitable for physiological research of the transplanted graft, e.g. functional tests, growth of the animal and metabolic changes.
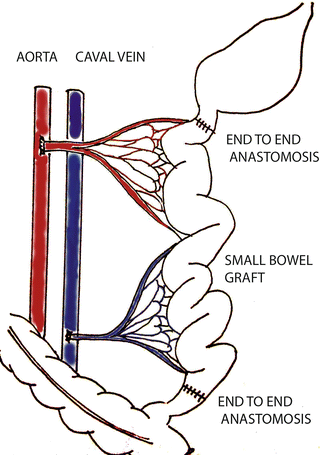

Fig. 13.2
Orthotopic SBT with systemic venous drainage (sketched by Dr. Petr Wohl)
13.2.3 Orthotopic SBT with Portal Drainage
This technique is similar to the above described technique with the difference being the venous anastomosis performed as a porto-portal connection (Fig. 13.3). Ischemia time during the clamping of venous anastomosis is an important factor for success of the procedure. If the time is longer than 15 min venostasis results in increased mortality. Even if this model represents the physiological way of venous drainage, in some studies the superiority over the systemic venous drainage was not proven [10, 11]. This model is always intended for special research such as pharmacokinetics of drugs metabolized after bowel resorption in the liver [2].
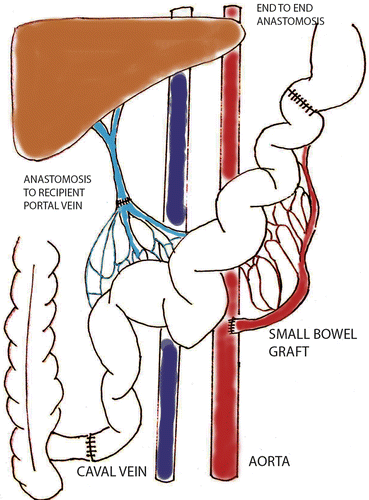

Fig. 13.3
Ortothopic SBT with portal drainage (sketched by Dr. Petr Wohl)
13.2.4 SBT with Using “Cuff Technique”
The standard surgical technique of small bowel transplantation using a “handmade” vascular anastomosis is a technically demanding microsurgical procedure requiring an experienced surgeon with long-time training. For this reason, SBT is always frustrating for the beginner associated with high mortality of experimental animals. The cuff technique is a simple method of microvascular anastomosis where the vascular connection of the polyethylene cuffs are inserted to the donor and recipient vessel and are then connected together (Fig. 13.4). The main advantage of this technique is the easy and fast performance of an unexperienced surgeon. There are exist some different types of this technique where the cuff is used for both anastomoses (arterial and venous) or only for venous anastomosis [3, 12]. The cuff technique is described in detail in Chap. 6.1.5 Vascular Anastomosis with Using “Cuff Technique”.
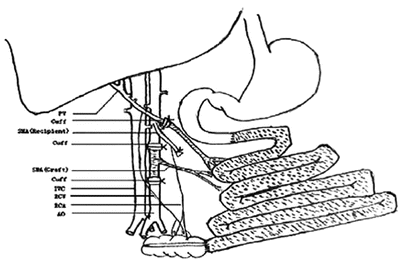

Fig. 13.4
Illustration of the complete reconstruction of the entire small bowel with using “cuff technique” [13]
13.2.5 SBT with Reconstruction of Lymphatic Vessel
This part of small bowel transplantation is described in Chap. 14.
13.3 Procurement of Small Bowel Graft
13.3.1 Surgical Equipment
Small bowel transplantation is performed under magnification using a surgical microscope. Instruments : scissors, forceps, micro-scissors (straight, curved), two micro-forceps (straight, curved), micro-needle holder. Material: 9-0 nylon suture, 7-0 and 4-0 silk suture, cotton swabs, gauze, saline solution, heparin solution, heating pad, needles, syringes.
13.3.2 Anesthesia
For general anesthesia a mixture of ketamine (10 mg/kg) with chlorpromazine (2.5 mg/kg) intramuscularly is recommended. Anesthesia offset can occur usually after 60–90 min and one third of the total dose should be administered to prolong it. The rat should be positioned on its back with easily extended extremities. The recommended magnification of surgical microscope is 12.5–20.0 times.
13.3.3 Surgical Procedure
1.
Perform a midline laparotomy.
2.
Retract the small bowel cranially toward the right side of the animal.
3.
Sharply dissect the avascular retroperitoneal membrane and divide the colon from the small bowel .
4.
Dissect the vasa ileocolica, vasa colica media and vasa colica dextra (in this step, separate the colon from the small bowel ).
5.
After repositioning the small bowel , identify the portal vein (Fig. 13.5).
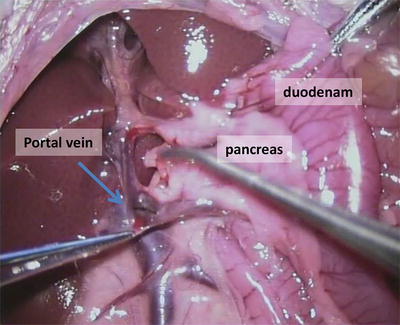

Fig. 13.5
Dissection of portal vein from the pancreas
6.
Dissect the vena pylorica with two silk ligatures and with a cotton swab gently divide the portal vein from the surrounding tissue and from the liver hilus toward to vena lienalis (Fig. 13.6).
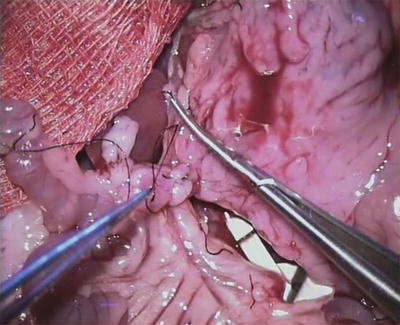

Fig. 13.6
State after dissection of the portal vein in the area of the liver toward to mesenterial root
7.
After assessment of appropriate length of small bowel , interrupt the mesenteric root so that the vascular supply for the graft will be preserved.
8.




Aorta preparation starts proximally from the aortic bifurcation (2 cm) by bluntly dissecting towards the stump of arteria mesenterica cranialis (amc). Divide the aorta from the surrounding tissue and from the vena cava caudalis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


