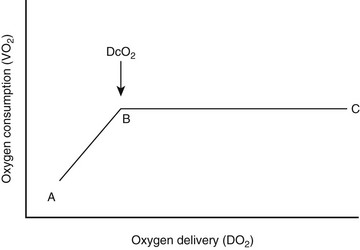
Chapter 4 Shock is a state of severe hemodynamic and metabolic derangements. It is characterized by poor tissue perfusion from low or unevenly distributed blood flow that leads to a critical decrease in oxygen delivery (DO2) in relation to oxygen consumption (VO2) (Figure 4-1) or inadequate cellular energy production. If the shock state is not promptly recognized and treated, neurohormonal compensatory mechanisms will lead to stimulation of the renin-angiotensin-aldosterone system, as well as baroreceptor- and chemoreceptor-mediated release of catecholamines and subsequent production of counterregulatory hormones (glucagon, adrenocorticotropic hormone [ACTH], and cortisol). These changes will increase cardiovascular tone, activate a variety of biochemical mediators, and stimulate inflammatory responses that contribute to the shock syndrome. This progression can cause or exacerbate uneven microcirculatory flow, poor tissue perfusion, tissue hypoxia, altered cellular metabolism, cellular death, and vital organ dysfunction or failure. Figure 4-1 The relationship between oxygen delivery and oxygen consumption. In region B-C, the oxygen consumption remains constant as oxygen delivery is increased. The oxygen supply is in excess of consumption and VO2 is termed supply-independent. During shock, as metabolic demand (VO2) increases or DO2 diminishes (C-B), OER rises to maintain aerobic metabolism so that consumption can remain independent of delivery. However, at point B, called critical DO2 (DcO2), the maximum OER is reached. This is believed to be 60% to 70%, and beyond this point any further increase in VO2 or decline in DO2 must lead to tissue hypoxia. (Data from Mellema M: Cardiac output, wedge pressure and oxygen delivery, Vet Clin North Am Small Anim Prac 31(6):1175, 2001.) Box 4-1 lists the different types of shock, but this classification can be overly simplistic. Critically ill patients are subject to complex etiologic and pathophysiologic events and therefore may suffer from more than one type of shock simultaneously. The rationale for the functional classification of shock is the presumption that each underlying illness identified can be associated with a specific pathophysiologic process and rapid, appropriate therapy can be administered. In cats the hyperdynamic phase of shock is rarely recognized. Also, in contrast to dogs, cats with shock have unpredictable changes in heart rate; they may exhibit tachycardia or bradycardia. In general, cats typically present with pale mucous membranes (and possibly icterus), weak pulses, cool extremities, hypothermia, and generalized weakness or collapse. In cats the lungs seem to be the organ most vulnerable to damage during shock or sepsis, and signs of respiratory dysfunction are common (Schutzer et al, 1993; Brady et al, 2000; Costello et al, 2004). Critically ill patients with inadequate tissue perfusion, DO2, or oxygen uptake often develop a hyperlactatemia and acidemia that reflect the severity of tissue hypoxia. Human patients with lactic acidosis are at greater risk of developing multiple organ failure and demonstrate a higher mortality rate (Nguyen et al, 2004). High blood lactate levels may also help to predict mortality in dogs (Boag and Hughes, 2005; de Papp et al, 1999; Nel et al, 2004; Lagutchik et al, 1998). The normal lactate level in adult dogs and cats is reported to be less than 2.5 mmol/L; lactate concentrations greater than 7 mmol/L are considered severely elevated (Boag et al, 2005). However, normal neonatal and pediatric patients may have higher lactate concentrations (McMichael et al, 2005). In addition, sample collection and handling techniques can affect lactate concentration (Hughes et al, 1999). Serial lactate measurements can be taken during the resuscitation period to gauge response to treatment and evaluate the resuscitation end points; the trends in lactate concentrations are a better predictor of outcome than are single measurements in both human and veterinary patients (Husain et al, 2003; Zacher et al, 2010; Green et al, 2011; Conti-Patara et al, 2012). The ability of the body to correct an elevated lactate concentration is directly correlated to survival. Ideally SvO2 is measured in a blood sample derived from a PAC because this represents a true mixed venous oxygen sample. However, in cases in which the insertion of a PAC is not possible or desirable, SvO2 can be determined within the central circulation using a central venous catheter in the cranial vena cava. SvO2 is then termed central venous oxygen saturation (ScvO2). In critically ill patients with circulatory failure of any origin, ScvO2 values generally are higher than SvO2, but the two measurements closely parallel one another. Therefore the presence of a low ScvO2 likely indicates an even lower SvO2. The difference between the two values is usually about 5% and can be explained by blood flow redistribution and differences in VO2 across the hepatosplanchnic, coronary, and cerebral circulations during shock states. The potential value of this monitoring was shown in a study comparing two algorithms for early goal-directed therapy in human patients with severe sepsis and septic shock (Rivers et al, 2001). In this study maintenance of a continuously measured ScvO2 above 70% (in addition to maintaining central venous pressure above 8 to 12 mm Hg, MAP above 65 mm Hg, and urine output above 0.5 ml/kg/hr) resulted in a 15% absolute reduction in mortality compared with the same treatment without ScvO2 monitoring (see Early Goal-Directed Therapy section later in the chapter for further details). ScvO2 measurement in small animals has not gained widespread use, but clinical research in critically ill and septic dogs suggests that it may prove useful as a diagnostic, prognostic, and monitoring tool in the future (Conti-Patara et al, 2012; Hayes et al, 2011). If the animal is not breathing or displays signs of impending respiratory fatigue, immediate intubation and positive-pressure ventilation should be instituted. If the animal is breathing spontaneously, oxygen is administered (see Chapters 10 and 11). This can involve flow-by methods (50 to 150 ml/kg/min) such as simply holding the oxygen tubing to the nose or administering oxygen into a mask, hood, or bag. Nasal or nasopharyngeal catheter(s) are effective methods of oxygen administration (using rates of 50 to 100 ml/kg/min per catheter). Once the vital signs are obtained, vascular access is established and fluid administration is initiated if indicated. Preliminary diagnostics and appropriate treatment should be rapidly initiated to maximize DO2 to the tissues and prevent irreversible shock.
Shock
Clinical Presentation
Patient Monitoring
Monitoring Perfusion and Oxygen Delivery
Blood Lactate Levels
Mixed Venous Oxygen Saturation and Central Venous Oxygen Saturation
Therapy of Shock
Oxygen
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine
