Fig. 1
Schematic presentation of important microbial transmission routes via which the human and (food) animals are in contact with each other. In blue control mechanisms are shown and in red some of the transmission routes that cannot be controlled, or escape control. Via the environment transmission may take place, through microbes present in excretion products and diseased animals, and in some countries also diseased humans. Next to the animals that are produced for direct consumption, in many developing countries animals are used as working animals to produce food and are thus included in this scheme. in these scheme. These animals when old or ill are often consumed (red dashed line), rather than destructed. Wildlife holds a broad spectrum of diseases including many highly pathogenic and deadly diseases. Though, the incidence may be low, because the associated risks are high, the consumption of wildlife animals and the spillover from wildlife to food/production animal is of importance. In Western countries wildlife is of lesser importance, however, in many developing countries and upcoming economies wildlife consumption may still be substantial, for instance in rural areas because of availability, as delicacies or other reasons. Moreover, in developing countries contact between humans and food animals may in general be easier and more frequent
The foodborne route is probably the most important gateway for this contact. The vast majority of infections with enteric zoonotic bacterial pathogens, such as Salmonella enterica, Campylobacter coli/jejuni, and Yersinia enterocolitica, probably occurs through this route. For other zoonotic pathogens, direct contact between animals and humans may also be an important route of transmission, this could be the case for Brucella spp., Enterohemorrhagic Escherichia coli (EHEC) or some newer strains of Methicillin Resistant Staphylococcus aureus (MRSA). Bacteria from production animals are widespread in the environment, mainly as a result of their presence in manure. Thus, the environment and wild fauna also transforms into reservoirs of foodborne pathogens and resistance, and forms a source of (resistant) pathogenic bacteria into the food animals and human reservoirs. Although consumption of wildlife is not considered a major route in many developed countries, wildlife is consumed at a substantial level in developing countries. In addition, because of generally lower bio-safety levels in rural animal keeping, contact between humans and food animals may in general be more frequent in these countries. For instance, the general understanding now is that the SARS epidemic in 2003 originated in direct human contact with and/or consumption of, wildlife, or indirectly through contact between wildlife and domestic animals (Guan et al. 2003; Shi and Hu 2008). Wildlife holds a broad spectrum of diseases including some of the most deadly ones. For this reason also the consumption of wildlife animals, and the spillover of infectious diseases from wildlife to food/production animal, is of global importance.
3 Food Animal Zoonoses in General
Although a number of very important zoonoses are related to—and in some cases directly transmitted from wildlife animals—the vast majority of zoonotic disease cases in the world actually relate to animals bred for food purposes. Such zoonotic pathogens include bacteria, such as Brucella, Salmonella, Campylobacter, verotoxigenic E. coli, and Leptospira, parasites, such as Taenia, Echinococcus, and Trichinella or virus, such as Influenza A H5N1 (Avian influenza) and Rift Valley Fever virus. It of course also includes ‘unconventional agents’, such as prions, of which the most well-known is the one causing Bovine Spongiform Encephalopathy (BSE) in cows and new variant Creutzfeldt-Jakob disease in humans.
Diseases originating on the farm can in many cases be efficiently dealt with on the farm. For example, brucellosis in animals (mainly cattle and sheep) has been eliminated in many countries, thereby virtually eliminating the human disease burden (Godfroid and Käsbohrer 2002). Also, some of the main parasites can be effectively controlled at the farm level, and this could work for both Taenia solium in pigs (defined by WHO/FAO/OIE as a ‘potentially eradicable parasite’), as well as, Trichinella spiralis (in many animals, including pigs); both have essentially been eliminated from farmed pigs in several northern European countries (WHO/FAO/OIE 2005; Gottstein et al. 2009).
3.1 Zoonoses Related to the Food Production Chain
Outbreaks of viral diseases in humans, originating in or spreading through farm animals (avian flu—H5N1 and ‘swine flu’—H1N1) have caused major global alerts in the last decade. These zoonotic, global influenza outbreaks (H1N1 even characterized by WHO as a pandemic) spread very quickly either in the animals (H5N1) or directly in the human population (H1N1). Although the total human disease burden related to the endemic bacterial zoonoses are probably manifold higher than these influenza outbreaks, it is basically these relatively few (but clearly global) outbreaks that have put One Health on the global agenda. The failure to predict or even monitor disease spread in animals in order to link this to the prevention of disease in humans, presented regulators, and politician with a wake-up call regarding the need for cross-sectoral collaboration between the animal and human health sectors.
In contrast to the dramatic viral outbreaks, bacterial food-related zoonoses are usually occurring endemically in farm animals. These pathogens are found in most food animals produced in industrialized settings. It should be realized that most countries—including most developing countries—have a significant part of the food animal production in some sort of industrialized setting. Such settings are invariably linked to a number of important zoonotic pathogens, including Salmonella, Campylobacter, and Escherichia coli. These pathogens, while widespread and endemic and in reality causing a major global disease burden, are not often recognized as important human pathogens. Part of the reason for this is the absence of a One Health framework, a framework that could ensure cross-sectoral collaboration and data-sharing and thereby lay the foundation for a realistic description of the situation, as well as, of potential sensible solutions. There are some countries (especially in northern Europe) that have instituted cross-sectoral data collection for zoonoses, typically through a construction called Zoonosis Centers. The data sharing across animal, food, and human health sectors have enabled science-based solutions, resulting most noticeably in significant reduction in human salmonellosis through lowering Salmonella prevalence in animals (Wegener et al. 2003). These constructions and solutions are clearly following One Health principles, and have basically done so since 1994! Similar solutions would be relevant in all countries with industrialized food animal production, but it is noteworthy that efficient solutions in this (sometimes called commercial) part of a national production system often will have repercussions also down to the traditional poor farmer (sometimes referred to as the communal production system). For instance, Salmonella enteritidis entered Zimbabwe through imported animals (poultry) in the commercial sector around 1993, and thereafter spread quickly to the communal sector, as well as, to the human population (Matope et al. 1998). The background for this is most likely that old animals from the commercial sector are sold to communal production systems. And where the animal goes the pathogen goes—therefore lowering the prevalence in the commercial sector would enable a reduction in prevalence also in the communal sector, thereby in turn lowering the human incidence of disease.
The spread of foodborne zoonoses through the food production chain has for more than 20 years been referred to as a ‘farm-to-fork’ (or ‘boat-to-throat’) issue related to the different stages of food production, but often originating at the farm (or all the way back to the feed used at the farm). This realization clearly represents original One Health thinking, and it should be noted that risk mitigation solutions under this framework typically have focused on a consideration of the full food production continuum, involving all relevant stakeholders. Figure 2 tries to capture a generalized picture of such a chain, starting with animal feed and ending in human consumption of animal-based food products.
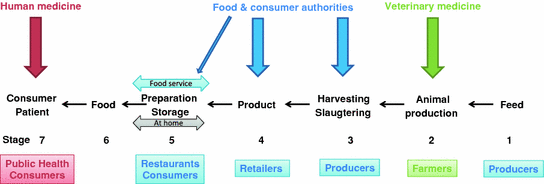

Fig. 2
Farm-to-fork scheme showing how infectious diseases may travel through the food chain. We have arbitrary defined seven stages that may be distinguished in the production chain of most animal derived foods. For individual food types, or non animals derived foods, different chains may be drawn in a similar way. Different controlling organizations are presented in the top of the picture and different stakeholders are presented in the lower part of the figure
3.2 Zoonoses Related to Poverty
Whereas zoonotic diseases with pandemic potential, such as avian or ‘swine’ influenza and SARS, have received committed attention from world leaders, and while zoonotic diseases related to industrialized food production systems have received some recognition leading to—at least in some countries—efficient risk mitigation action, a number of very important zoonotic diseases, disproportionately affecting poor and marginalized populations, are largely ignored.
These types of zoonoses are many, and the prevalence in animal populations vary according to local agricultural, demographic, and geographic conditions. For many such diseases solutions to dramatically decrease the disease burden are well-known, but action is lagging (for example, for many of the parasitic zoonoses). WHO refers to such diseases as ‘Neglected Diseases’ (Molyneux et al. 2011).
The group of Neglected Zoonoses include bacterial diseases, such as brucellosis (with significant sequelae), leptospirosis, Q-fever (with high mortality), and bovine TB. Bovine TB appears to be increasing in many poor settings with HIV infections as an important factor for progression of TB infection to active TB disease. For both, brucellosis and bovine TB the disease in cattle causes lowered productivity, but seldom death, and both infections have been largely eradicated from the bovine population in the developed world, by a test-and-slaughter program, thereby in effect eliminating this human health problem (Godfroid and Käsbohrer 2002).
Important zoonotic parasitic diseases include schistosomiasis, cysticercosis, trematodiasis, and echinococcosis, several of which with significant mortality rates or long-term sequelae including cancer and neurological disorders. Cysticercosis is emerging as a serious public health and agricultural problem in poor (García et al. 2003). Humans acquire Taenia solium tapeworms when eating raw or undercooked pork meat contaminated with cysticerci. The route of transmission is, pigs are infected through Taenia eggs shed in human feces, and the disease is thus strongly associated with pigs raised under poor hygienic conditions. This again means that the cycle of infection can be relatively easily broken when introducing efficient management, as has been the case in most developed countries.
Given that 70 % of the rural population in poor countries is dependent on livestock and working animals to survive (FAO 2002), the effect of these animals carrying a zoonotic disease can be dramatic, both relative to human health directly, but also as it affects the potential to earn an income. This also affects the potential mitigation action; for instance the large-scale culling of animals, which can be a viable solution in rich countries, might be problematic in the poorest countries. Such solutions would mean not only loss of food, but also a serious socio-economical disruption, in some cases leading to national instability.
4 AMR in Food Animals
In the early 1940s, antibiotics were first introduced to control bacterial infections in humans. The success in humans led to their introduction in veterinary medicine in the 1950s, where they were used in both production and companion animals. Next to agricultural animals, antibiotics, nowadays, have also found their way into intensive fish farming and some are used to control diseases in plants. Their use is thus wide-spread.
Antibiotics in animals are used essentially in three ways: for therapy of individual cases, for disease prevention (prophylaxis) treating groups of animals, and as antibiotic growth promoters (AGP). For AGP use, antibiotics are added to animal feed at sub-therapeutic concentrations to improve growth. The mechanism by which this works was (and still is) unclear, nevertheless, this type of antibiotic use led to a steep increase in antibiotic consumption when it was introduced. In general, when first introduced, the use of antibiotics led to improved animal health and most likely to higher levels of both food safety and food security. The use of antibiotics therefore sky-rocketed. Between 1951 and 1978, the use in the United States alone went from 110 to 5580 tons (WHO 2011).
However, the use of antibiotics in animals has over the years also resulted in a selective pressure for AMR microorganisms, contributing significantly to the human health problem of AMR bacteria; notably a number of bacterial strains that were previously susceptible to antibiotics are now, in very high frequencies, becoming resistant to these antibiotics, some of them representing very important or even last resort treatment potential for humans (Bonten et al. 2001). Nowadays, there are serious efforts by national authorities and some international organizations to reduce the antibiotic overuse in animal production (WHO 2011; FAO/OIE/WHO 2003), especially—but not only—through abolishing their use as AGP. However, there seems to be major problems in ensuring cross-sectoral understanding, and indeed cross-sectoral solutions in this area. The veterinary profession and the medical profession is seen as accusing each other of AMR problems, and in a sense they are both right—all use of antimicrobials can cause AMR, therefore both animal and human use cause problems. But in order to achieve a science-based understanding of the problem, we need data on both animal and human uses, and about both AMR in bacteria in animals, in food, and in humans. Therefore, a One Health approach in which all stakeholders work together will be necessary to investigate the problems and provide science-based solutions that can efficiently reduce the spread of AMR bacteria from animals to humans (most often through food) and vice versa (most often through the environment).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


