CHAPTER 1 Respiratory Infections
EQUINE RESPIRATORY TRACT
Normal Respiratory Flora
Bacterial flora plays an important role in host health in a variety of tissues and organ systems, such as the skin, gastrointestinal tract, and urogenital system, as well as the respiratory system.1 The upper airway of healthy horses contains many bacteria, including a variety of aerobic and anaerobic species. This flora competes with pathogenic species that, when present in large numbers, can colonize the epithelial surface. Normal equine respiratory flora includes Streptococcus equi subsp. zooepidemicus, Pasteurella spp., Escherichia coli, Actinomyces spp., and Streptococcus spp. Anaerobes predominate in the normal equine oral cavity and consist of several bacterial genera, including Bacteroides fragilis, Fusobacterium spp., Eubacterium spp., Clostridium spp., Veillonella spp., and Megasphaera spp.2
Typically, horses with infectious lower airway disease are infected with one of these bacteria, consistent with the concept that contamination of the lower respiratory tract originates from the upper airways. Aspiration can be the mechanism by which such contamination occurs, because head elevation and long-distance transport contribute to lower airway contamination and accumulation of mucus.3–5 Contamination of the lower airway is common in apparently healthy horses, and many horses will have positive bacterial cultures when examined by tracheobronchial aspirate.
Pulmonary Defense Mechanisms
A major mediator of nonspecific clearance of debris and pathogens from the respiratory tract is the mucociliary escalator. The mucociliary escalator consists of a double layer of mucus that extends from the pharynx to the respiratory bronchioles. This mucous layer is propelled upward by the ciliated respiratory epithelium. Inhaled particles and debris are propelled in a proximal direction by a constant wave of upward movement by the cilia and mucus. The mucociliary system can become damaged by smoke inhalation and direct viral destruction.6,7
Influenza and herpesviruses replicate within and destroy ciliated epithelium, which requires approximately 21 days for regeneration. In addition, environment may play a role in clearance mechanisms because high ammonia concentration, such as that associated with a high degree of urine and fecal waste, will result in depressed ciliary motility.8–10 Dehydration may also contribute to reduced pulmonary clearance because effective ciliary movement will be depressed with reduced fluidity of the mucous layer.
The major mediators of specific pulmonary clearance mechanisms are within the bronchial-associated lymphoid tissue (BALT). BALT exists within the submucosa of the segmental bronchi and terminal bronchioles. As with other lymphoid organs, BALT is an area where antigen-specific responses stimulate cell-mediated and humoral immune defense. B lymphocytes within BALT can switch to all classes of antibodies, although the predominant antibody produced in the upper respiratory tract is immunoglobulin A (IgA); immunoglobulin G (IgG) is secreted in greater quantities in the lower airways.11 The advantage of upper respiratory secretion of IgA is the blockage of adherence of pathogens to the upper respiratory tract epithelium, a process referred to as immune exclusion. Memory is conferred by this arm of the immune system and thereby results in long-term protection from infectious disease.
Below the level of the mucociliary escalator and the BALT, cellular responses are critical for immune protection of the equine host. The first phagocyte of importance is the alveolar macrophage, located in the terminal bronchioles and alveoli. The alveolar macrophage provides a bridge between innate and adaptive immune responses. Although these cells can ingest foreign material nonspecifically, they also serve as important antigen-presenting cells for T lymphocytes and development of adaptive immunity. Particles that are inhaled and reach the alveolar spaces are removed by local alveolar macrophages. Once they contain foreign material, these cells may be coughed up and swallowed, or they may move from the alveolar space and enter general circulation for ultimate clearance by the lymphatic system. The function of these cells depends on host status; long-distance transport or viral infection will destroy alveolar macrophages.12
Another important cell in the pulmonary system of horses is the pulmonary intravascular macrophage (PIM). These cells are important for removal of particulate matter (e.g., bacteria, toxins) from general circulation. Species that have PIMs include horses, pigs, ruminants, and cats. Mammalian species that do not have PIMs utilize hepatic Kupffer’s cells and splenic macrophages for similar purposes. PIMs are critical for removal of bacteria or endotoxin on the first pass through the lungs, but they contribute to the inflammation induced after bacterial challenge.13 Disadvantages of PIMs include the resultant inflammatory reaction that follows their activation. For example, phagocytosis of endotoxin is associated with pronounced inflammatory mediator release, microthrombus formation, neutrophilic influx, vasoconstriction, pulmonary edema, and endothelial damage that may lead to other systemic disorders. Species variation in sensitivity to endotoxin relates to the presence of PIMs, and intensified sensitivity to endotoxin is related to the number of PIMs in the pulmonary vasculature.
Epithelial protection of the respiratory tracts is provided not only by the mucosal lining and leukocytes within the submucosa, but also by mechanisms of the innate immune system. Antimicrobial peptides play an important role in innate immune protection in the pulmonary system of many species. Cathelicidin peptides have been identified in pulmonary equine neutrophils collected from heaves-affected individuals.14 This class of antimicrobial peptide has been shown to play a role in pathogen clearance, having broad-spectrum activity against bacterial pathogens.15 Other similar peptides are expressed in epithelial cells as well as within leukocyte subsets.16,17
DIAGNOSTIC APPROACH TO INFECTIOUS RESPIRATORY DISEASE
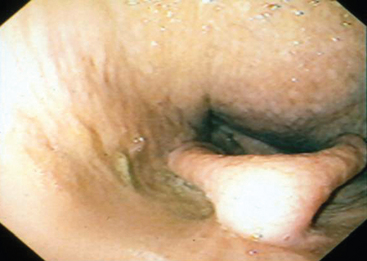
Endoscopy may be used to confirm the presence of upper airway disease and to perform diagnostic tests such as transtracheal aspirate (TTA) or bronchoalveolar lavage (BAL). Diagnosis of rhinitis, pharyngeal lymphoid hyperplasia, guttural pouch disease, and retropharyngeal lymphadenopathy (presumably from Streptococcus equi subsp. equi infection; Fig. 1-1) may be facilitated by endoscopic examination. Radiographs of the sinuses are essential for identification of fluid or masses within sinuses, guttural pouches, and thorax. Diagnostic-quality thoracic radiography is difficult with most field equipment. Lower airway radiography is important for determining the type of pattern present but is not specific for identification of any particular etiologic agents. Thoracic ultrasound is easily performed in the field, and although nonspecific for etiology, it is particularly useful to detect lower airway disease, including consolidation of the peripheral lung lobes and identification of pulmonary fluid. A 5.0-MHz linear probe (used for most rectal ultrasound examinations) will suffice for the majority of these examinations.
Diagnosis of equine influenza is based on virus isolation, virus antigen detection, and paired serum testing (see Chapter 12). An ELISA test is commercially available for the detection of influenza virus particles in nasal secretions (Directigen FLU-A, BD Diagnostic Systems, Franklin Lakes, NJ). This potential stall-side test was not designed for equine use but has been validated for use in this species.18
Diagnosis of infection with equine herpesvirus (EHV)-1 and EHV-4 depends somewhat on disease manifestation (see Chapter 13). For example, diagnosis of infectious respiratory disease may be confirmed by PCR or culture of nasal swab samples (or buffy coat samples) or detection of increasing serum antibody titers to the virus. Virus isolation from a buffy coat smear, nasal swab, or postmortem tissues may provide important information. Additional diagnostic testing may include molecular characterization using the restriction endonuclease analysis of deoxyribonucleic acid (DNA) fragments. Cerebrospinal fluid (CSF) analysis should be performed in horses suspected of EHV myelopathy, often characterized by xanthochromia and albuminocytologic dissociation. Antibody titer analysis of CSF is of limited value for diagnosis of neurologic EHV disease because significant disruption of the blood-brain barrier has frequently occurred in affected horses.
Diagnosis of EHV-2 may be challenging because virus isolation does not provide consistently positive results. Paired serologic titers may provide suggestive information for establishing a diagnosis of upper respiratory disease in horses. PCR has also been used for diagnosis in horses.19
Definitive diagnosis of equine viral arteritis (EVA) infection is made on the basis of virus isolation from nasopharyngeal, vaginal or semen samples (see Chapter 14). PCR testing has improved sensitivity and specificity for detection of the virus in these samples. Acute and convalescent serum titers may provide additional information to facilitate confirmation of the EVA diagnosis in suspect cases. Gross examination of fetuses postmortem typically reveals edema, pleural effusion, and petechiation.
Equine rhinoviruses, such as equine rhinitis A virus (ERAV), are a cause of upper respiratory tract infection in horses (see Chapter 16). These infections can be more challenging to diagnose than other viral respiratory tract infections because seroprevalence can be high.20 Of 28 cases where the rhinovirus was isolated from infected horses only six showed serologic evidence of viral exposure.21 Therefore, when equine rhinitis B virus (ERBV) infection is a diagnostic differential, virus isolation is the preferred diagnostic test. Although a third rhinovirus, ERBV2, has been investigated as a possible etiologic agent of equine viral respiratory tract disease, its role remains unconfirmed.22
Culture of nasopharyngeal swabs or wash samples are useful for diagnosis of Streptococcus equi subsp. equi (strangles) infection in horses (see Chapter 28). Detection of S. equi DNA using the PCR test is also confirmatory for a respiratory infection secondary to S. equi infection. Carrier horses may be challenging to identify without endoscopic evaluation that includes examination of the guttural pouches. Culture and PCR of samples obtained from the guttural pouch of these horses are recommended. If both tests are negative, the horse is unlikely to be an S. equi carrier. PCR testing for S. equi is more sensitive than standard microbiologic culture techniques.23
UPPER RESPIRATORY TRACT INFECTIONS
Rhinitis and Sinusitis
Nasal airways can be infected with a variety of viral, bacterial, fungal, and parasitic agents with resultant sinusitis or rhinitis. In this chapter, rhinitis in the horse is defined as infection of the nasal passage independent of the sinus. Infection may include the nasal concha but does not involve the conchal sinuses unless caused by viral agents. Specific viral agents include equine influenza virus, EHV-1 and EHV-4, equine rhinoviruses, and adenovirus.20,22,24–29 Bacterial rhinitis is uncommon and usually occurs secondary to trauma or a foreign body. Mycoplasma spp. have been isolated at postmortem examination from horses with rhinitis.7,21,29,30 A variety of mycotic agents, such as Aspergillus spp. (see Chapter 56), Conidiobolus spp. (usually C. coronatus) (see Chapter 55), and Cryptococcus neoformans (see Chapter 57), may cause rhinitis in horses.31–34 The most common cause of parasitic rhinitis is myiasis resulting from Habronema, Draschia (see Chapter 62), and the Russian gadfly, Rhinoestrus purpureus.35 Enzootic lymphangitis or glanders caused by Burkholderia mallei causes a specific granuloma within the sinus cavity (see Chapter 39).36–42
Horses with sinusitis most often have unilateral disease, unless the infection is viral or there is extensive involvement of the nasal septa. Most horses present with respiratory stridor and nasal discharge with diminished airflow.24,26,28,29 Therapy usually involves surgical debridement, debulking of nasal granuloma, and local therapy for the specific agent.28,43–45 Orally administered itraconazole has been described for treatment of recurrent nasal mycoses.28,44 Although successful in this case, the pharmacokinetics of itraconazole are variable. Fluconazole may be a viable alternative. Detailed discussions of antifungal therapy in horses are presented in Chapters 56 and 71.
Sinonasal disease is very common in the horse. The horse has six pairs of sinuses, including the conchal sinuses, which exchange air with the nasal airway. The frontal sinuses may be affected with granulomatous masses (usually fungal or parasitic) or empyema (bacterial). The most common bacterial isolates are Streptococcus spp., with S. equi subsp. zooepidemicus and S. equi subsp. equi most frequently found. Staphylococcus spp. are the next most common isolates.46–49 Mixed bacterial infections may occur. Cryptococcus neoformans and Coccidioides immitis also may cause granuloma formation within the paranasal sinuses31–34 (see Chapter 51).
In a study of 277 horses with sinusitis, 24% had primary sinusitis with no history of predisposing trauma or dental infection.49 Dental disease of the third to sixth maxillary cheek teeth was the most common predisposing factor for secondary sinusitis (22% of horses), followed by sinus cysts, neoplasia, progressive ethmoidal hematoma, trauma, mycotic infection, sinonasal polyps, and nasal epidermal inclusion cysts. Primary infection of a rostral maxillary cheek root infection was identified in only 4% of cases, although computed tomography (CT) evaluation was not used for diagnosis in many of these horses. Nasal discharge (most often unilateral but occasionally bilateral) and facial swelling were the most common clinical signs. Discharge can be mucopurulent to serosanguineous fluid with a foul smell. Clinical signs frequently persisted over several weeks (without other progressive systemic clinical signs). Other signs of frontal and maxillary sinus involvement included lacrimal discharge and exophthalmia.
Diagnostic techniques that may facilitate identification or characterization of sinusitis in horses include endoscopy, radiography, CT, and magnetic resonance imaging (MRI).50–52 Endoscopy can detect changes in airway structure (84% of cases) and rule out ethmoidal hematoma.48,49 Sinoscopy can also be performed through a space created in the skull by trephination in the standing horse.26,31,48,49
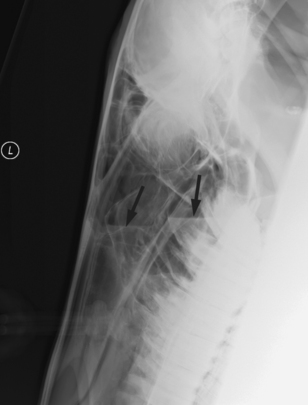
Radiography is essential for identification of fluid and masses within sinuses (Fig. 1-2). If there is no fluid, this modality is valuable for detection of tooth root abscess.47,49–55 Usually the first molar is involved. CT and MRI are exceptionally valuable for detection of tooth root involvement and bony changes, which often involve the maxillary bone and facial crest.
Appropriate and effective treatment of sinusitis requires that underlying or predisposing conditions be accurately identified and treated, that debris be flushed from the sinus, and that associated infectious agents be properly identified. When fluid is present within a sinus, medical treatment with antibiotics alone is unlikely to be successful. Trephination and flushing or surgical debridement and drainage through a sinus flap are indicated.28,44,45,48,56–59 Establishment of ventral drainage of the affected sinuses may be required. Local flushing is likely to be the most important component of therapy, although systemic antimicrobial therapy may be indicated for any horse with signs of osteomyelitis.
Prognosis is guarded for complete resolution of clinical signs, especially when apical dental disease is present. Frequently, tooth removal is indicated. Recurrence is most common with ethmoidal hematoma and neoplasia.49
Lymphoid Pharyngeal Hyperplasia
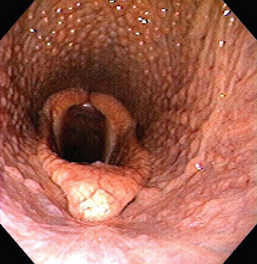
Lymphoid pharyngeal hyperplasia is a common condition involving the upper respiratory tract of 2- and 3-year-old racehorses (Box 1-1 and Fig. 1-3). Most mild cases respond favorably to reduced athletic activity combined with systemic and topical antiinflammatory therapy. Dexamethasone can be administered at a dose of 0.02 to 0.05 mg/kg orally daily for 1 week, followed by half the original dose orally for 1 week, then the same dose orally every other day for an additional week. A throat spray of nitrofurazone, dexamethasone, and dimethyl sulfoxide is reported to be of benefit when administered topically.56 Systemic immune modulation is reported to be effective for treatment of horses with lower airway inflammation and may also have some benefit in those with upper airway inflammation.56,60,61 Occasionally, chronic disease occurs; reports have suggested that these horses may respond favorably to cautery of the dorsal roof of the pharynx.62
Box 1-1 Grading Scheme for Lymphoid Pharyngeal Hyperplasia
Modified from Raker CW: The nasopharynx. In Mansmann RA, McAllister ES, editors: Equine medicine and surgery, Santa Barbara, Calif, 1982, American Veterinary Publications.
American Veterinary PublicationsOrganisms associated with a more prolonged course of pharyngeal hyperplasia include S. equi subsp. equi, equine influenza, EHV-1, EHV-2, and EHV-4. The condition is thought to result from chronic inflammation of the localized lymphoid tissues, particularly because these structures have a diffuse distribution within the mucosa in this species. Although some investigators have cultured the oropharynx of affected horses, no consistent etiologic agent has been identified. Normal inhabitants of the equine upper respiratory tract, such as S. equi subsp. zooepidemicus, Bordetella bronchiseptica, and Moraxella have been isolated; however, the direct association with lymphoid pharyngeal hyperplasia has not been determined. A grading system has been established for this condition; those horses with more severe inflammation have greater numbers of bacterial organisms isolated from their upper respiratory tract.63
Arytenoid Chondritis
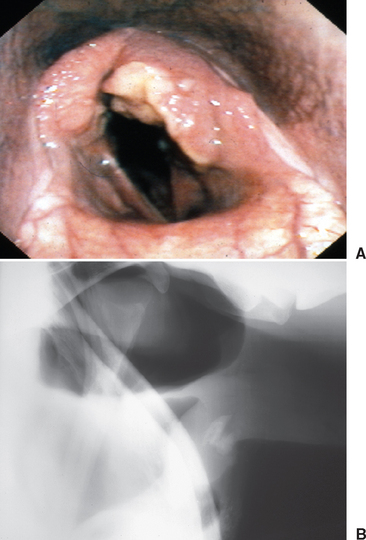
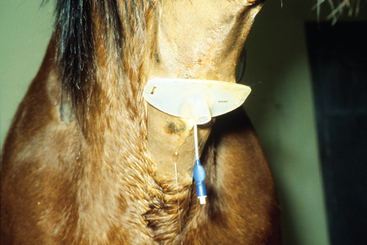
Arytenoid chondritis is a progressive inflammatory condition of the arytenoid cartilages in adult horses, originating as an infectious condition. Most often, upper airway dysfunction is reflected in poor athletic performance and respiratory stridor. Diagnosis is based on upper airway endoscopy (Fig. 1-4). One manifestation of chondritis is the development of granulomas on the axial surface of the arytenoid cartilages. Clinical management of affected patients involves medical or surgical therapy. Although broad-spectrum antibiotic therapy has been attempted in many cases, it is rarely curative. Also of importance in the management of some horses with arytenoid chondritis is placement of a tracheostomy tube (Fig. 1-5). Several techniques are described for placement of a permanent tracheostomy.6
Viral Diseases
Equine influenza virus is classified as an orthomyxovirus with a single-stranded, segmented ribonucleic acid (RNA) genome64 (see Chapter 12). Influenza viruses are classified on the basis of surface and internal protein antigens into three types: A, B, and C; only type A influenza is reported to infect horses. Major viral antigens include neuraminidase (NA) and hemagglutinin (HA). Two type A viral subtypes are known to cause disease in horses: H7N7 and H3N8.64 The strain H7N7 was initially isolated in 1956 in Prague and designated A/equine/Prague/56. This H7N7 variant, termed equine-1 influenza, has not been isolated since 1980 and is believed to have disappeared from the equine population.63 The H3N8 equine virus, called equine-2 influenza, was initially isolated in Miami in 1963 and designated A/equine/Miami/63.65–67 Antigenic drift has subsequently resulted in many subtypes of variant equine-2 among horses, including A/equine/Fontainebleau/79, A/equine/Kentucky/81, A/equine/Saskatoon/90, and A/equine/Newmarket 2/93. Although antigenic drift has been observed for many years, antigenic shift, a larger-scale change in the antigenic nature of the equine influenza viruses, has not been documented to date.
Influenza is most common in horses commingled under stressful conditions, such as race training.67 Infection occurs through inhalation of viral particles. The virus infects respiratory ciliated epithelium, leading to loss of the mucociliary escalator for pathogen and particle clearance. Therefore, viral infection predisposes affected individuals to secondary bacterial infection.
Equine herpesviruses are classified among the alpha (EHV-1, -3, and -4) and gamma (EHV-2 and EHV-5) herpesviruses (see Chapter 13). The viruses associated with respiratory disease of the greatest significance in the horse are EHV-1 and EHV-4. These α-herpesviruses are responsible for sporadic respiratory disease, abortion, and myeloencephalopathy. (EHV-4 is less likely to be associated with disease other than respiratory infection.) Fatal neonatal sepsis has been reported in association with EHV-4 infection, but EHV-1 is more often involved in this form of disease.69,70 Because EHV is neurotropic, latency is possible and generally associated with the trigeminal nerve (cranial nerve V) ganglion or lymphocytes.71 During times of severe stress or immune suppression, disease transmission is possible when latent virus is reactivated.
Clinical signs of disease associated with EHV typically include pyrexia, serous nasal discharge, and occasionally cough. In addition to respiratory disease, late-term abortion, neonatal sepsis, and myeloencephalopathy may also result from infection with EHV.63,69–72
Equine herpesvirus-2 (EHV-2), often referred to as cytomegalovirus, is a slow-replicating virus that typically results in self-limiting viral respiratory disease in young horses.63 Many investigators question the role of this virus as a primary etiologic agent of fulminant respiratory disease in horses. EHV-2 has been recovered from both normal horses and young horses with clinical signs of respiratory tract disease.73–76 Studies of seroprevalence and virus isolation reveal that young foals are often exposed to the virus. The virus could also be isolated from fluid collected by transtracheal aspirate of young horses with clinical respiratory tract disease, whereas it was rare to isolate the virus from tracheal fluid of clinically normal foals. Experimental inoculation of foals with EHV-2 results in chronic pharyngitis.77 This organism may be a pathogen of concern in predisposing foals to bacterial pathogens such as R. equi.73
Although most often recognized for its association with the equine reproductive tract, equine viral arteritis (EVA) causes a mild to moderate respiratory disease (see Chapter 14). The virus is maintained in equine populations in carrier stallions because testosterone is required for persistence and maintenance of the virus in vivo. Carrier stallions maintain the virus within the ampulla and vas deferens. Clinical manifestations of EVA are similar to those of other viral respiratory tract diseases. Variations in the severity of clinical signs result from strain differences in virulence, pathogen dose, and host immune function. Incubation requires several days to 2 weeks, with a more rapid course of disease after venereal transmission. Clinical signs associated with respiratory tract infection include serous nasal discharge, submandibular lymphadenopathy, mild to moderate cough, and ventral edema. Most infections are self-limiting, although edema may be severe and respiratory distress evident. Abortion typically occurs within a month of exposure and disease development. Abortion may occur, although other clinical signs of EVA have not been observed. Neonatal foals infected with the virus demonstrate respiratory difficulty and rarely recover from viral infection.78–80 Hematologic evidence of EVA includes leukopenia, characterized by a lymphopenia, and thrombocytopenia, which may be severe.
Equine rhinoviruses have been divided into two serogroups: equine rhinitis virus A and B (ERAV and ERBV) (see Chapter 16). ERAV is grouped in the Aphthovirus genus based on genotype and similarity with other members of this genus, such as foot-and-mouth virus, as well as the characteristic viremia and persistent shedding that occurs following infection with the virus.19,27 ERBV is the sole member of the genus Erbovirus. A third serotype has been identified with the proposed classification in the erboviruses as ERBV2; currently this virus is referred to as P13/75 and is classified in the family Picomaviridae.22 Clinical manifestations of disease typically include pyrexia, serous to seromucous nasal discharge, coughing, depression, anorexia, pharyngitis, and submandibular lymphadenopathy.81 Mild lymphopenia and increased plasma fibrinogen concentrations have been reported in affected horses.
Strangles
Clinical disease in horses associated with Streptococcus equi subsp. equi infection (strangles) is most common in horses younger than 5 years, but very uncommon in young foals (<3 months) born to mares previously exposed to the organism (see Chapter 28). Natural infection results from direct contact with an infected or carrier individual that may have overt clinical disease or that has maintained the organism within the upper airway, most frequently the guttural pouch.58 Transmission may also occur through fomites, such as on contaminated clothing or cleaning instruments.
Infection with S. equi primarily occurs through oral and nasal routes. Incubation from time of infection to manifestation of clinical signs varies from a few days to a few weeks and is influenced by pathogen virulence, dose of inoculum, and host immunity at the time of challenge. Some of the earliest clinical signs include fever, depression, and reduced appetite. Nasal discharge may initially be serous but with disease progression will become mucopurulent. Lymph node enlargement and abscess maturation generally require approximately 7 days to occur. Early in the course of infection, affected lymph nodes are sensitive to palpation and firm in nature. As rupture becomes imminent, a soft center develops, and a serous crust on the surface may be observed. Submandibular and retropharyngeal nodes are most often affected; edema may be severe, resulting in dysphagia and respiratory stridor. After rupture of abscess(es), swelling will diminish rapidly. Severe obstruction may necessitate that a tracheostomy be performed.
LOWER RESPIRATORY TRACT INFECTIONS
Etiology and Epidemiology
Miscellaneous Causes of Pneumonia
Infectious disease involving the lower respiratory tract is most often associated with bacterial infection, although fungal 32–34,82,83 and viral71,84,85 pathogens are also potential invaders of the lower respiratory tract. Septic thrombophlebitis is considered a risk factor for metastatic spread of septic foci.86 Some reports suggest that the presence of anaerobic organisms warrants a more guarded prognosis when cultured from horses with pleuropneumonia.87 Polymicrobial infection may result from synergy among pathogens, particularly aerobes or facultative anaerobes and anaerobic organisms that favor survival of organisms that otherwise would not proliferate.
Pulmonary Abscess
Pulmonary abscess formation most often occurs in weanling-age foals in association with Rhodococcus equi infection (see Chapter 32). Streptococcus equi subsp. zooepidemicus is the organism most frequently cultured from the lungs of horses with generalized pneumonia and rarely results in abscess formation. Complications from S. equi subsp. equi infection include metastatic spread to various organs, including possible pulmonary abscess formation. Aspiration is another cause of focal pulmonary infection and abscessation. Aspiration pneumonia is a potential complication of esophageal obstruction or dysphagia in horses. Neonatal foals may develop dysphagia in association with hypoxic ischemic encephalopathy or nutritional muscular dystrophy, whereas adult horses may develop aspiration pneumonia after complete esophageal obstruction.
Pathogenesis
High-level performance horses may be predisposed to lower airway infection because alveolar macrophages are reduced in efficacy after strenuous exercise.12 In addition, challenge is enhanced because horses in training are at greater risk for aspiration of pathogens and particulate matter. Horses used for performance activities often travel long distances, and persistent head elevation, as might occur in a trailered horse, reduces pulmonary clearance mechanisms within 6 to 12 hours.3 Although many strategies have been used to enhance protection in such individuals, neither antibiotic therapy nor intermittent lowering of the head appears to reduce substantially the incidence of pulmonary infection.87 Transportation for a distance greater than 500 miles in the preceding 2 weeks is an important risk factor for the development of pleuropneumonia in horses. Although uncommon, horses with severe gastrointestinal disease or those with pulmonary infection that remains unresponsive to antibiotic therapy may develop a pulmonary mycotic infection.34 Diagnostic testing should be implemented to rule out fungal organisms as primary or secondary invaders.
Clinical Findings
Clinical findings in horses with pulmonary disease most often include depression, fever, and reduced food intake. Coughing is most frequently observed during physical exertion or with advanced disease. Horses with advanced disease may show respiratory distress as well as pronounced weight loss. Purulent nasal discharge with a fetid odor, evidence of thoracic pain, and epistaxis may occur in association with rupture of a pulmonary abscess. A sequela to severe disease may be laminitis; therefore, abnormal gait or intermittent recumbency may accompany evidence of pulmonary infection.
Therapy
Treatment of horses with historical, clinical, and hematologic evidence of pulmonary bacterial infection should include broad-spectrum antimicrobial therapy (pending sensitivity testing of isolates and implementation of more targeted antimicrobial therapy) and excellent supportive care. Although bacterial culture results are not available immediately on diagnosis of pulmonary infection, cytologic evaluation of pulmonary aspirates should give the clinician some indication of the type (Gram stain) and population (single vs. multiple classes of pathogens) of pathogens in the patient. Beta-lactam antibiotics combined with aminoglycosides provide good coverage for a variety of pathogens that may infect the lower airways of horses. Caution should be used in individuals that are debilitated or dehydrated.71 Because of nephrotoxicity, aminoglycosides are contraindicated for use in patients at risk for renal impairment.
PNEUMONIAS
Pleuropneumonia
Etiology and Epidemiology
Pleuropneumonia is a condition in which infection associated with bronchopneumonia has spread to involve the pleura and the pleural space.88 This disorder most often occurs in performance horses, frequently after long-distance transport.63,71,89 Although apparently spontaneous cases of pleuropneumonia may occur in some horses, most affected horses have experienced one or more predisposing risk factors, such as long-distance transport, recent viral or bacterial respiratory tract disease, or a recent episode of general anesthesia.87,90,91
Most cases of equine pleuropneumonia result from bacterial infection, but reports also demonstrate that Mycoplasma spp.,30 viral agents,21 and mycotic agents34 may be isolated, or the disease may occur as a complication of septic thrombophlebitis.86 Rarely, pulmonary hydatidosis may be a cause of equine pleuropneumonia92 (see Chapter 61). Bacterial pleuropneumonia can be associated with a single pathogen but more often results from a mixed infection that may include aerobic and anaerobic organisms.87,91,93,94 The most important factor for the development of transport-associated pleuropneumonia is head position during long-distance transport.87,89,95–97 The most compelling evidence for this claim is the observation that horses transported long distances without restraint of head position do not develop changes in lower airway cytologic findings. In contrast, horses without other stress had an estimated 75% increased likelihood of developing lower airway accumulation of bacteria and inflammatory debris after a minimum of 24 hours of head restraint.3,87,88,91 High-intensity exercise in combination with long-distance transport further contributes to development of lower airway inflammation and impaired immune clearance mechanisms.12
Striving to prevent equine pleuropneumonia is important because the prognosis for return to previous level of athletic function may be guarded to poor in severe cases.87,91 Complications associated with pleuropneumonia, such as laminitis and chronic abscess formation, may negatively influence the future athletic performance of affected individuals.87
Clinical Findings
Thoracic auscultation usually reveals abnormal pulmonary sounds. When pleural effusion is present, the most common finding is attenuation of audible bronchovesicular sounds over the ventral lung fields. In the dorsal lung fields, normal pulmonary sounds may be heard; more often, however, increased bronchovesicular sounds are heard, accompanied by crackles or wheezes. Before significant pleural fluid accumulation, abnormal pulmonary sounds may be heard diffusely.98,99 With chronicity, friction rubs are common, reflecting fibrin accumulation along the parietal and visceral pleural surfaces.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree