Chapter 32 Reptile and Amphibian Analgesia
The last 2 decades have seen improved understanding of the mechanisms of nociception and importance of treating pain in vertebrates of all classes. The treatment of pain has become standard practice in veterinary medicine, because there is a moral imperative to treat pain and alleviation of pain has been found to facilitate anesthetic recovery and healing in animals.6 At one time, it was assumed that lower vertebrates were less capable of perceiving pain and distress, but the current understanding is that pain perception in these species is likely to be analogous to that in mammals.11 In a survey of 367 members of the Association of Reptile and Amphibian Veterinarians, 98.4% of respondents stated that they believe that reptiles and amphibians feel pain.15 Despite this, only 39.5% regularly provided analgesia to their patients. Veterinarians treating reptiles and amphibians have historically used empirical dosing and anecdotal observations to guide treatment of pain. Recently, methods for measuring antinociception have been successfully adapted to amphibians and reptiles, allowing data for these taxonomic groups to expand (Table 32-1). Despite these advances, large gaps remain regarding analgesic efficacy, pharmacokinetics, and toxic side effects for many commonly used analgesics. Although the long-term goal is to develop specific dosages for all classes of reptiles and amphibians, those charged with the care of these animals must make judicious use of current knowledge to treat pain.
Pain Pathways and Mechanisms: the Case for Pain
Pain is the result of exchanges among three major components of the nervous system, the peripheral nerves, spinal cord, and brain. The basic anatomic, physiologic, and biochemical components of pain pathways that exist in mammals also exist in nonmammalian species.1,8 In amphibians, four distinct afferent nerve fibers have been identified that are analogous to those found in mammals to transmit different types of noxious stimuli.11 Afferent nerve fibers release neurotransmitters that activate neurons in the spinal cord, which then transmit electrical impulses to the thalamus region of the brain. In humans, the thalamus sends pain messages to three specialized regions of the brain. The somatosensory cortex identifies and localizes pain, the limbic system is responsible for emotional response and the experience of suffering, and the frontal cortex assigns meaning to pain. The limbic and cortical brain may then modulate the nociceptive signal with a number of endogenous substances that may act to increase or decrease the pain experience. Amphibians and reptiles do not have the well-defined limbic cortex and neocortex typical of later evolved species, and the thalamocortical connections in frogs appear diffuse and poorly organized compared with those of mammals.9 How these anatomic differences affect the perception of pain is unknown. Research has shown that reptiles and amphibians have similar endogenous mechanisms to modulate pain and that analgesia may be achieved through the use of pharmacologic agents used to treat pain in other species.1,18,20–22
Dosing Principles and Side Effects
The diversity found within the reptile and amphibian classes is very broad, and analgesia research has been conducted only on a very limited number of species. Drugs with species-specific pharmacokinetic data and proven efficacy should be selected when possible; however, extrapolation is often necessary. As with other classes of drugs, clinicians rely on knowledge of basic physiology to inform empirical dosing. The metabolic rate of reptiles and amphibians is lower than that of mammals and, as poikilotherms, it is critical that they are maintained at their preferred optimum temperature zone during treatment. Dehydration and malnutrition may affect drug absorption and clearance and should be corrected prior to treatment, especially when using potentially nephrotoxic or hepatotoxic drugs. The site of drug administration may also affect pharmacokinetics. It has been shown that a substantial proportion of blood from the hindlimbs of turtles may drain directly to the liver.7 Consequently, drugs that are extracted by first pass through the liver may have lower systemic availability when injected into the hindlimb, as observed in red-eared sliders being dosed with buprenorphine.10
Veterinarians are often interested in providing analgesia at the time of anesthesia. Reptiles and amphibians undergoing biopsy, endoscopy, surgical treatment for trauma, or other procedures expected to be painful should be provided with analgesia. In general, it is recommended that analgesia be provided before a painful procedure (preemptive analgesia) to avoid sensitization of the nervous system and amplification of pain. In reptiles and amphibians, analgesics may need to be administered well before a procedure to achieve therapeutic levels. Monitoring anesthetic depth and parameters of cardiovascular and respiratory function may be difficult in amphibians and reptiles. Clinicians may be concerned that administering opioids or α2 adrenergics will depress cardiovascular or respiratory reflexes, or prolong anesthetic recovery. A study comparing cardiovascular effects and blood gas changes during isoflurane or isoflurane and butorphanol anesthesia in the green iguana has found that preemptive or intraoperative use of butorphanol is unlikely to be detrimental to cardiac function.12 It is of note that in both study groups, animals were mechanically ventilated during the study and the effects on respiration were not measured. Therefore, more research is needed to determine the affect of providing analgesia at the time of anesthesia.
Reptile Research Findings
Chelonia: Turtles and Tortoises
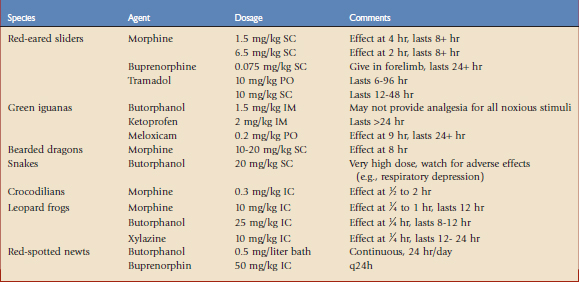
Among neuropeptides, the opioids are the most potent analgesics and are classified according to their receptor subtypes, including mu, delta, and kappa receptors. Few studies have been undertaken to determine which opioid receptor types are involved in analgesia in reptiles. Mu and delta receptors are located throughout the brain in red-eared sliders. The delta receptors are more abundant; however, it is thought that they play a lesser role in antinociception.18 Butorphanol is considered the most widely administered analgesic in reptiles; therefore, demonstrating efficacy and determining dosages is a priority.15 Red-eared slider turtles were used in a study to determine the thermal antinociception efficacy of morphine and butorphanol.19 Morphine administered IM at low (1.5 mg/kg) and high (6.5 mg/kg) doses significantly increased hindlimb withdrawal latencies, showing effects after 2 to 4 hours that lasted at least 8 hours postadministration. The higher dose provided more rapid onset but otherwise similar results. At 24 hours postadministration, some increase in withdrawal latencies remained, but the results were not significant. An unexpected finding was that butorphanol at a dose of 2.8 or 28 mg/kg provided no thermal antinociception in red-eared sliders. Morphine is primarily a mu agonist with delta and kappa activities, and butorphanol is a kappa agonist and mu antagonist. Additional trials using selective agonists for each opioid receptor type have led to the conclusion that in red-eared sliders, thermal antinociception is a result of mu receptor activation.18 Kappa receptor activation, either with a receptor-selective opioid or with butorphanol, was not found to produce thermal antinociception.
Buprenorphine is a synthetic opioid with partial agonist action at the mu receptor and antagonist actions at the other opioid receptors. In humans, buprenorphine is about 20 to 30 times more potent than morphine as an analgesic, is long-acting, and is associated with less respiratory depression. Plasma levels of 1 ng/mL are associated with effective analgesia in human surgery patients. After administration of 0.05 mg/kg SC, 85% of red-eared slider turtles maintained this target plasma level for 24 hours.10 Pharmacokinetic data have suggested that this plasma level is expected to be achieved for at least 24 hours after SC administration of a dose of 0.075 mg/kg in the forelimb of red-eared sliders. Injection in the hindlimb provided lower plasma levels, suggesting that there is substantial first-pass metabolism by the liver.
A study was conducted to elucidate the effects of opioid analgesia on respiration. It was determined that both morphine and butorphanol cause respiratory depression in red-eared sliders by decreasing breathing frequency.19 Tidal volumes were not affected and were actually increased with butorphanol. Ventilatory suppression was more marked and longer lasting with morphine.
The analgesic and respiratory effects of tramadol have also been investigated in the red-eared slider. Tramadol has a dual mechanism of action and provides analgesia by acting at mu opioid receptors and inhibiting the reuptake of norepinephrine and serotonin. Analgesic effects were measured using hindlimb withdrawal latencies to thermal stimuli. Oral doses ranging from 1 to 25 mg/kg and SC doses of 10 and 25 mg/kg were tested.2 Tramadol, 10 mg/kg PO, increased latencies for 6 to 96 hours after dosing compared with 12 to 48 hours when given subcutaneously. When given by either route at a dose of 25 mg/kg, mouth gaping and muscle flaccidity were seen. Respiratory depression was seen at all oral doses over 5 mg/kg. A pharmacokinetic study of the NSAID meloxicam in red-eared sliders has shown that intramuscular dosing is the most effective way to provide predictable blood levels by avoiding rapid clearance (seen with IV dosing) and secondary peaks (seen with PO dosing).16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree