CHAPTER 8 Reproductive Tract Infections
Infections of the reproductive tract cause a myriad of clinical disorders manifesting primarily as infertility, abortion, and birth of septic foals. This chapter discusses the etiology, pathogenesis, clinical manifestations, treatment, and prevention of these diseases in the mare and stallion. Infectious processes of the reproductive tracts may be divided into venereal and opportunistic. The venereal diseases present important economic and regulatory issues and are reviewed separately in this chapter.
REPRODUCTIVE TRACT INFECTION IN NONPREGNANT MARES
Infection of the reproductive tract in the mare, especially endometritis, is the leading cause of infertility in horses and results in substantial annual losses. The female reproductive tract possesses a variety of mechanisms to protect itself against infection. These include physical barriers (vulva, vestibulovaginal sphincter, cervix), local immune mechanisms, and the physical ability to eliminate products of inflammation. Uterine infections, reported in 25% to 60% of barren mares,24,111 become established when one or several of these natural defense mechanisms fail or become overwhelmed. Bacterial infections result in infertility, early embryonic loss, placentitis, birth of septic foals, and postpartum metritis. Salpingitis, cervicitis, and vaginitis may be part of the clinical presentation of acute or chronic reproductive tract infections in the mare.
Uterine Infections
Etiology
Infectious endometritis is a major cause of infertility and early pregnancy loss* and is estimated to affect 25% to 40% of broodmares.109 These infections become established when normal defense mechanisms fail to clear potentially pathogenic organisms after they are introduced into the uterus.282 The most common sources of uterine contamination include coitus, parturition, artificial insemination, and aseptic genital examination and manipulation.149,381,385 Age, parity, number of barren years, and uterine biopsy grade influence likelihood of persistence of infection.76,333
The organisms most frequently isolated from mares with endometritis are Streptococcus equi subsp. zooepidemicus, Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae.149,356,385 Infections caused by P. aeruginosa or K. pneumoniae are often considered venereal diseases because the organisms are often introduced during coitus, insemination with infected semen, or genital manipulations. K. pneumoniae capsule types 1, 2, and 5 are highly pathogenic.307
Commensal bacteria such as Actinomyces pyogenes, Proteus spp., and Staphylococcus spp. are occasionally isolated from mares with endometritis. They are considered likely to be the causative organisms of endometritis if the diagnosis is supported by cytologic or histopathologic evidence of concurrent inflammation. Alpha-hemolytic Streptococcus, Enterobacter spp., and Staphylococcus epidermidis are rarely causes of equine endometritis and should be considered simple contaminants.79 Corynebacterium spp. and anaerobic bacteria such as Bacteroides fragilis may occasionally cause endometritis in horses.6 Anaerobes are most often isolated from postpartum and foal-heat samples.335 Taylorella equigenitalis is the causative agent of a highly contagious venereal metritis, contagious equine metritis (CEM) (see Chapter 41).
The pathogenicity of bacteria depends on their ability to adhere to the endometrium, preventing their removal by normal uterine clearance mechanisms. Adhesive proprieties of S. equi subsp. zooepidemicus are probably mediated by fibronectin-binding proteins and hyaluronic acid capsule.130,199,232,433 Attachment of K. pneumoniae to endometrial cells is facilitated by pili and capsule.129 Persistent colonization by P. aeruginosa is assisted by the secretion of an adhesive matrix that forms a biofilm.291 These biochemical proprieties also are important in resistance of bacteria to opsonization, phagocytosis, and the action of antimicrobials.96,113,284,433 Resistance to phagocytosis is often observed with S. equi subsp. zooepidemicus and K. pneumoniae and is probably mediated by antigenic variation, antiphagocytic M-like proteins, hyaluronic acid capsule, or polysaccharide and Fc receptors.113,433 Bacterial toxins promote deterioration of complement and exacerbate uterine inflammation.243
Candida spp. and Aspergillus are the most common fungal organisms isolated from the uterus of mares with endometritis.385 The incidence of fungal infection in mares with endometritis is estimated to vary between 0.1% and 5%.99,100,120,318,444 Fungal organisms isolated from the equine uterus include Aspergillus spp., several Candida spp., Cryptococcus neoformans, Fusarium spp., Hansenula anomala, Hansenula polymorpha, several Rhodotorula spp., Scedosporium apiospermum, Saccharomyces cerevisiae, Trichosporon beigelii, and Torulopsis candida.*
Prolonged antibiotic therapy may be a predisposing factor for yeast overgrowth.444 The use of antibiotic-containing semen extenders for artificial insemination may be partially responsible for the apparent increase in the number of mares with fungal endometritis (J. Aurich, personal communication). Transmission of fungal organisms from stallions has not been demonstrated, although fungi have been cultured from the urethra (Mucor spp.), fresh semen (Absidia spp.), and extended semen (Candida spp.) of stallions.252
Mycoplasma spp. have been isolated from the external genitalia and semen of clinically normal and infertile stallions, but their exact role in uterine infection is not well established.273,367,448 In vitro, Mycoplasma equigenitalium produces a consistent cytopathic effect on the ciliated epithelium of the oviduct, with variable severity depending on strain.31 M. equigenitalium, M. subdolum, and Acholeplasma spp. are associated with infertility, endometritis, vulvitis, and abortion in mares and with reduced fertility and balanoposthitis in stallions.167,201,203,273 M. equigenitalium and M. subdolum were isolated from the genital tract of mares (5%-34%) and aborted equine fetuses (7%); however, the presence of mycoplasmas is not always correlated with reduced fertility.30,167,201,203,273
Pathogenesis
Physical Barriers.
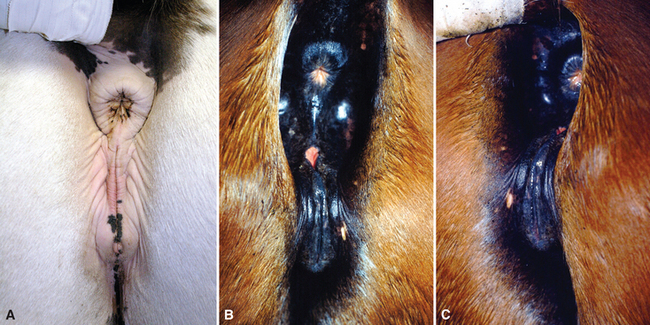
The uterine cavity is protected from ascending infection by several anatomic structures.237 The first line of defense is provided by the seal of the normal vulvar labia. Evaluation of vulvar and perineal conformation should be included in all prebreeding examinations or evaluations for infertility. The vulva should be in a vertical position aligned with the anal opening (Fig. 8-1, A). The labia should be tight, with most of its length below the tuber ischii. The vulvar lips may become parted as a consequence of malformation caused by previous traumatic injuries or lesions (Fig. 8-1, B). In older multiparous mares, there is a tendency for extreme relaxation of the vulvar lips, particularly during estrus, as well as tilting of the dorsal aspect of the vulva caused by relaxation of the perineal body. In this situation the vulva becomes horizontal as it is pulled cranially over the tuber ischii (Fig. 8-1, C). These anatomic changes predispose the mare to pneumovagina (windsucking) and pneumouterus, ultimately causing urine to pool in the cranial vagina and contaminate the uterus when the cervix is open. Contamination with fecal material adds to the increased risk of infection.
Immunity and Uterine Defense.
The concept that mares may be innately “resistant” or “susceptible” to uterine infection was introduced in the 1960s based on the ability of mares to clear experimentally induced or naturally acquired bacterial endometritis.183,299 Young mares experimentally inoculated with S. equi subsp. zooepidemicus clear infection within a few hours,183 whereas barren mares inoculated with S. equi subsp. zooepidemicus and P. aeruginosa have a delayed elimination of bacteria.299 These observations led to further investigation into uterine defense mechanisms to clarify the role of local immunity, neutrophil function, opsonization, and phagocytosis in the prevention and clearance of uterine infection.
Immunoglobulins.
The predominant immunoglobulins in uterine secretions are IgG and IgA produced within the endometrium.15,196,272, 434–436,438 Uterine immunoglobulin concentration does not differ between mares susceptible and resistant to endometritis, suggesting that this is not a major factor in susceptibility to infection.* In fact, susceptible mares tend to have slightly higher concentrations of intrauterine immunoglobulins than do resistant mares, but susceptible mares are less efficient at opsonizing streptococci during acute infection.15
Neutrophils.
Neutrophil chemotaxis is induced by bacteria, endotoxin, spermatozoa, semen extenders, and even sterile water and saline. A massive influx of neutrophils into the uterine lumen occurs in both susceptible and resistant mares15,18 after local exposure to foreign proteins.18,424,439,440 In some mares, this stimulation elicits a persistent inflammatory response after breeding that has been termed persistent mating-induced endometritis (PMIE). Neutrophils play an important role in this phenomenon, and their effects are exacerbated if bacteria are present.254,391
The events leading to PMIE are initiated by a local reaction to the primary antigen, with local production of inflammatory mediators, especially prostaglandin E2 (PGE2), and neutrophil influx.372 Increased vascular permeability resulting from proinflammatory mediators exacerbates the neutrophil influx and leakage of serum proteins into the uterus, peaking as early as 4 hours after inoculation.
This response is primarily mediated by inflammatory mediators such as leukotriene B4 (LTB4) and PGE2.43,422,424 Mares susceptible to PMIE have a higher expression of proinflammatory cytokines (interleukin-1 beta [IL-1β], IL-6, and tumor necrosis factor alpha [TNF-α]) during estrus and IL-1β and TNF-α during diestrus.142 It is unclear whether chemotactic responsiveness differs between PMIE-susceptible mares and nonsusceptible mares.235,236,419 A second influx of inflammatory fluid is seen 12 hours later. Resistant mares are able to eliminate most fluid by 12 hours in response to the effects of oxytocin and prostaglandin on the myometrium. Susceptible mares fail to eliminate fluid, often because of inherent endometrial or myometrial pathology that renders uterine contractions less efficient in uterine clearance.
The phagocytic activity of neutrophils is thought to be enhanced on entry into the uterine cavity.419,422 Phagocytic activity is higher in ovariectomized mares treated with estrogens,423 suggesting that neutrophil phagocytic activity may be highest during estrus. Phagocytic activity of circulating neutrophils is no different between susceptible and resistant mares; however, phagocytic activity and life span of uterine neutrophils are significantly reduced in susceptible mares.16,83,422,446 Susceptible mares are more likely to have uterine clearance problems and accumulate more fluid, which may contribute to a reduction in the viability of neutrophils. Differences in neutrophil function between mares susceptible and resistant to endometritis have been demonstrated.234–236
Opsonizing activity in the uterus peaks 8 hours after inoculation with streptococci.163 Studies using heat-treated uterine fluid suggest that complement is not a primary opsonizing factor in the uterus.62,163 Complement is cleaved in uterine fluid, reducing its opsonizing ability within the uterine environment.14,62 The uterine environment of endometritis susceptible mares seems to be hostile to complement.18,62,219,422 In contrast, the opsonizing capacity of serum and degree of serum complement activity does not differ between susceptible and resistant mares.14,18,44,62,416 These observations provide a rationale for the use of serum infusion for the treatment of endometritis.14,292
Physical Clearance of Infection.
Physical clearance of pathogens and inflammatory debris from the uterus plays a major role in the prevention of persistent infection and is most effective during estrus.* Younger mares are able to eliminate both antigenic (S. equi subsp. zooepidemicus) and nonantigenic (microsphere) particles more quickly than older mares.127 Mares susceptible to infectious endometritis are unable to eliminate bacteria from the uterus in the immediate postovulatory period.222,397,398
The effect of uterine pathology on susceptibility to endometritis has been demonstrated.334 Gross anatomic changes, such as large pendulous uteri, defective myometrial activity, pendulant broad ligaments, and degenerative changes to the vascular and lymphatic drainage of the uterus, are also involved in delayed uterine clearance and the pathogenesis of endometritis.194,224,228 Microscopic alteration of the endometrium (ulceration, degeneration, or lack of cilia) may be involved in failure of mucociliary clearance.80,130,131,139,334
Myometrial contractions are less frequent and of shorter duration and intensity in susceptible mares,337,399 perhaps because of increased fibrosis or biochemical factors affecting uterine contractility. Uterine contractions are reduced in the presence of nitrous oxide (NO), which is found in high concentration in susceptible mares.7 The role of prostaglandin secretion in PMIE remains unclear despite numerous investigations, possibly reflecting variation in factors inherent to the endometrium that cannot be easily controlled in experimental studies.28,70,71
Diagnosis
Accumulation of large quantities of fluid during estrus or postbreeding is a good indication of mare susceptibility to endometritis (Fig. 8-2). Resistant mares eliminate mating-induced endometritis within 6 to 12 hours after breeding, whereas susceptible mares may retain variable amounts of fluid for several days. Accumulation of fluid in the uterus is associated with decreased pregnancy rates. All mares should be evaluated 24 hours after breeding and treated if a pocket of fluid remains within the uterine cavity.25,255,278,320 Mares with a history of infertility or that are known to be susceptible to endometritis should be evaluated or treated as early as 6 hours after breeding.392
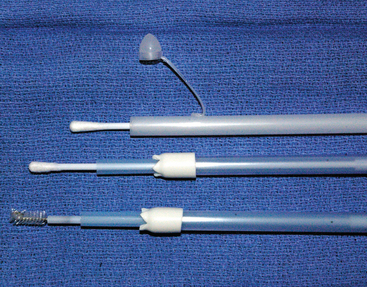
Several methods for collection of uterine cytology samples have been described. These include the use of double-guarded swabs, uterine cytology brushes, and low-volume uterine flushes.74 In the authors’ opinion, uterine cytology brushes and small-volume uterine flushes provide the best specimens for endometrial cytology (Fig. 8-3). Swabs are often reliable if there is fluid in the uterus or if the swab is wetted with a sterile saline solution before sampling.
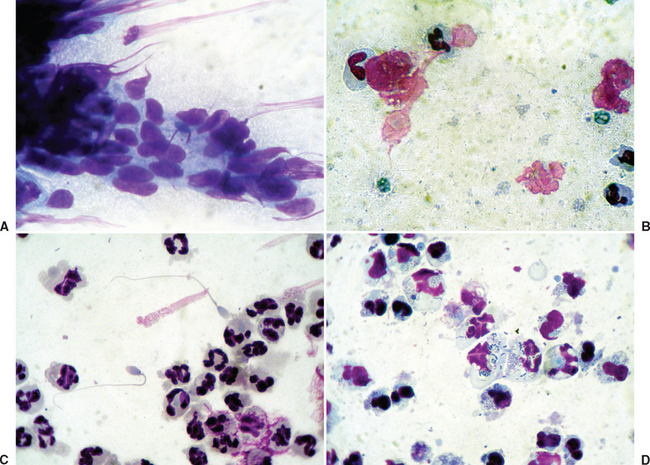
Interpretation of uterine cytology samples may be confusing because of the lack of standardized methodology for evaluation. The most common staining technique for uterine cytologic specimens is a rapid Wright-Giemsa stain (Diff-Quik). Interpretation is based on evaluation of the type of cells and organisms observed74,75 (Fig. 8-4). Quantitative methods for the interpretation of equine endometrial cytology have been reviewed recently.74 Diagnosis of endometritis may be based on the number of neutrophils per number of high-power microscopic fields, as a ratio of neutrophils to epithelial cells, or as a percentage of all cells. Endometrial cytology may provide an important clue to fungal infection. Cytology may be negative if infection is recent.100
Endometrial swabs should always be submitted for anaerobic and aerobic bacterial as well as fungal culture. Blood agar plating may be used in the initial fungal culture, but specific culture techniques are required for more precise diagnosis.100
Endometrial biopsy is an important and highly accurate method for the diagnosis of uterine inflammation. Special staining techniques, such as Gomori’s methamine silver, are particularly helpful for the diagnosis of fungal endometritis.140
Interpretation of diagnostic results may be challenging at times. Bacteriologic culture and cytology from endometrial biopsy are most accurate (high sensitivity and positive predictive value) for diagnosis of endometritis. The sensitivity of bacteriologic culture from endometrial biopsy is 82%; diagnosis based on cytologic and bacteriologic examination of endometrial swabs has a sensitivity of 77% and 34%, respectively.279
Therapy
Uterine Lavage.
The goal of uterine lavage is to “clean” the uterine cavity of organisms, dead neutrophils, cell debris, and products of inflammation before local antimicrobial therapy.13,209,401 Uterine lavage may enhance uterine contractions and clearance as a result of transient irritation of the endometrium.447 Uterine lavage is performed using warmed, balanced electrolyte solution (e.g., lactated Ringer’s, physiologic saline) with or without added antiseptics. A new solution specifically formulated for flushing of the equine uterine (Equine Uterine Flush, AB Technology, Pullman, Wash) is adjusted to appropriate pH and osmotic pressure and contains surfactants to aid in the removal of organisms and debris. Uterine lavage is preferably performed using a large Foley catheter in the same manner as for embryo collection (Table 8-1).
Table 8-1 Guidelines for Uterine Flushing and High-Volume Uterine Lavage
| UTERINE FLUSHING | HIGH-VOLUME LAVAGE (POSTPARTUM) |
|---|---|
| Indications | |
| Chronic endometritis, pyometra | Partial or total placental retention |
| Endometritis | Metritis |
| Persistent mating-induced endometritis | After obstetric manipulation/fetotomy |
| After uterine prolapse | |
| Equipment | |
| Silicone Foley long catheter (28 or 34 French, 100-mL cuff) | Stomach tube; make sure that tube has several side openings (1-1.5 cm in diameter). |
| Flushing bag (2.5 L) | Bucket |
| Stomach pump | |
| Mare Preparation | |
| Sedation if needed. | Often needed. |
| Wrap tail in plastic sleeve. | Wrap tail in plastic sleeve. |
| Palpation per rectum to evacuate feces. | Palpation per rectum to evacuate feces. |
| Clean perineal area and vulva. | Clean perineal area and vulva. |
| Place catheter using sterile sleeve and lubricate. | Place nasogastric tube deep into uterine cavity (within chorioallantoic cavity if placenta is retained). |
| Insert catheter into cervix and inflate balloon, making sure it is snug against anterior cervical os. | Protect orifices of tube by cuffing hand around it. |
| Fluid Choice | |
| Warm saline (least preferred) | Warm saline |
| Warm lactated Ringer’s solution (LRS) | Warm distilled water + 34 g of table salt per liter |
| Proprietary fluid (e.g., Equine Uterine Flush lavage solution) | Warm LRS (may be too expensive) |
| Warm tap water with 5 mL of 10% povidone-iodine solution and 8.5 g of table salt per liter | |
| Fluid Delivery and Monitoring | |
| Gravity using flushing bags | Pumped directly into uterine cavity |
| Volume varies from 500 mL to 2 L | Up to 15 L (depends on size of uterus) |
| Palpate transrectally to make sure that both uterine horns are sufficiently distended before emptying. | Flush twice a day if mare retained placenta or mare is sick. |
| Repeat flushing until return is clear. | Repeat flushing daily as needed (until placenta is passed if retained). |
| Monitor fluid retained by ultrasonography or measuring fluid collected. | Monitor by transabdominal ultrasound. |
| Evacuation Help | |
| Before and after flushing: oxytocin (10-20 IU intramuscularly) | Retained placenta (20 IU oxytocin every 4 hours in first 12 hours) |
| Postbreeding flushing should be started no earlier than 4 hours after breeding. | Exercise mare. |
The addition of antiseptics such as povidone-iodine or chlorhexidine to uterine lavage fluid is occasionally recommended for the treatment of equine endometritis. However, there is some concern about the effect of these antiseptics on neutrophil function. At high concentration, antiseptics may cause severe inflammation (necrosis) and irreversible damage to the endometrium, with evidence of discomfort at the time of infusion.406 Povidone-iodine solution may be used at a concentration of 0.05% (5 mL of 10% povidone iodine solution in 1 L of balanced salt solution) to facilitate elimination of bacterial infection. Adverse reactions are observed in mares after infusion with solution at a concentration of 1% or more.57 Uterine lavage with 0.05% solution of povidone-iodine solution 4 hours after breeding did not adversely affect pregnancy rates.59 Use of chlorhexidine diacetate is contraindicated in mares.17 Chlorhexidine gluconate may cause vulvar inflammation and vaginal straining at concentrations as low as 0.5% and endometrial inflammation at concentrations of 0.25%.186
Lavage with EDTA-TRIS (1.2 g NaEDTA and 6.05 g TRIS/L of H2O, titrated to pH 8.0 with glacial acetic acid) 3 hours before infusion of antibiotic has been recommended for treatment of persistent uterine infection with Pseudomonas spp. Ethylenediaminetetraacetic acid (EDTA) is thought to bind Ca++ in bacterial cell walls, making cell walls permeable to antibiotics and bacteria more susceptible to bactericidal activity of antibiotics.442
Antimicrobials.
The aim of local uterine infusion of antimicrobials is specifically to eliminate the causative organism35,233,385 (Table 8-2). The choice of antibiotic is dictated by results of endometrial culture and sensitivity, predicted antimicrobial efficacy in the uterine environment, and consideration of possible adverse uterine effects. Nonbuffered or precipitating solutions should be avoided. Streptococcus equi subsp. zooepidemicus and E. coli, two of the most common bacterial causes of endometritis, are sensitive in vitro to amikacin and gentamicin.356 The selection of antimicrobial for intrauterine infusion should take into account the pH and solubility of the drugs as well as the solution used for infusion. Some antibiotics, such as ceftiofur, will lose most of their activity if diluted in a saline solution.332 The volume of infusion should be sufficient to treat the entire uterine cavity, usually between 60 and 120 mL. Aqueous solutions of sodium benzypenicillin, neomycin, polymixin, and furaltadone are generally safe and useful for treatment of horses with acute endometritis.320,332 Aqueous solutions of penicillin, ampicillin, carbenicillin, ticarcillin, ticarcillin and clavulanic acid, kanamycin, and neomycin have also been recommended. Low-pH antimicrobials such as gentamicin and amikacin should be buffered with an equal volume of 7.5% sodium bicarbonate before infusion.17,58,393
Table 8-2 Antimicrobials for Treatment of Endometritis in Mares
| DRUG | DOSE* | COMMENTS |
|---|---|---|
| Gram-Positive Bacterial Infections | ||
| Penicillin sodium of potassium salt | 5 million units (U) | Very effective for streptococci; economical. |
| Ampicillin | 1-3 g | Can be very irritating; use at high dilutions; sodium salt precipitates on endometrium that remains in uterus for prolonged period. |
| Carbenicillin | 2-5 g | Reserved for persistent Pseudomonas (synergistic efficacy with aminoglycosides); usually given on alternate days with aminoglycosides; slightly irritating. |
| Gram-Negative Bacterial Infections | ||
| Gentamicin sulfate | 500-1000 mg | Highly effective; generally nonirritating when mixed with an equal volume of NaHCO3 and diluted in saline. |
| Amikacin sulfate | 2 g | Use for Pseudomonas, Klebsiella, and persistent gram-negative infections. |
| Kanamycin sulfate | 1 g | Toxic to spermatozoa; do not use close to breeding. |
| Polymyxin B | 1 million U | Particularly effective against Pseudomonas. |
| Neomycin sulfate | 3-4 g | Use for sensitive E. coli; can be irritating; do not use near time of breeding. |
| Gram-Positive and Gram-Negative Bacterial Infections (broad spectrum) | ||
| Cephazolin sodium | 1 g | |
| Ticarcillin | 1-3 g | Use for Pseudomonas; do not use for Klebsiella. |
| Ceftiofur sodium | 1 g | Once daily either intramuscularly or by intrauterine infusion. |
| Fungal (Yeast) Infections | ||
| Nystatin | 0.5-2.5 million U | Primarily for yeast (e.g., Candida albicans) in the growing phase; insoluble, suspend in 100-250 mL sterile water and vigorously mix immediately before infusion. |
| Amphotericin B | 100-200 mg | For infections with Aspergillus, Candida, Mucor, or Histoplasma; dilute in 100-250 mL sterile water, a relatively insoluble suspension. |
| Clotrimazole | 500-700 mg | For yeast infections (Candida spp.); crushed tablets mixed with 40 mL sterile water. |
| Fluconazole | 100 mg | For Candida spp. infections. |
| Miconazole | 200 mg | Most efficacious for yeast infections (Candida spp.) and some resistant fungal infections; infuse once daily for up to 10 days; dilute in 40-60 mL sterile saline before infusion. |
* All doses are for intrauterine infusion unless otherwise indicated.
Uterine infusion of enrofloxacin was an effective treatment for endometritis in 80% of 17 mares.144 Antimicrobial concentrations in endometrial tissues were greater than the minimum inhibitory concentration (MIC) for most bacterial pathogens for up to 24 hours after intrauterine infusion of enrofloxacin at a dosage of 2.5 mg/kg.143 Moderate endometrial inflammation was observed 24 hours after infusion but resolved progressively within 2 weeks.
Treatment of fungal endometritis requires large-volume uterine flushes followed by intrauterine therapy with antimycotic drugs for 7 to 10 days. Treatment for a longer duration may be required for some mares. The drugs most often used are polyene antimicrobial agents (e.g., amphotericin B, nystatin, natamycin), which alter membrane permeability, and imidazole derivatives (e.g., clotrimazole, econazole, ketoconazole, fluconazole, itraconazole), which interfere with nutrient exchange across the fungal cell wall and cell membrane.99 The prognosis for fertility after treatment of fungal endometritis remains generally poor because of the histologic changes resulting from the chronic inflammation.444
Lufenuron, a benzoylphenyl urea derivative and inhibitor of chitin synthesis, used for flea control in dogs and cats, has been recommended for treatment of mares with Candida parapsolosis, Candida paratropicalis, and Aspergillus fumigatus uterine infections. The inhibition of fungal growth is thought to be caused by disruption of the chitin-rich cell wall that surrounds these organisms. Intrauterine lavage is performed with lufenuron (540 mg) suspended in 60 mL of sterile saline solution.174
Systemic antimicrobials (e.g., amikacin,63 gentamicin,297 ticarcillin,366 procaine penicillin G, ampicillin, potentiated sulfonamides,17 ceftiofur sodium242) have been used for the treatment of endometritis, but little is known about drug concentrations obtained within the endometrium or treatment efficacy after systemic administration. Systemic treatment has the advantage of preventing inadvertent recontamination of the uterus and repeated trauma to the vagina and cervix in mares. Ciprofloxacin (2.5 g/day) and probenecid (1 g/day) were recommended for systemic treatment of Pseudomonas infection.393 Although an oral dose of ciprofloxacin of 0.5 mg/kg has been used by some practitioners,61 pharmacokinetic studies have shown that bioavailability of the drug is not very high.119 Fluoroquinolone antibiotics such as enrofloxacin and ciprofloxacin reach therapeutic concentrations in endometrial tissue after intravenous (IV) administration.2,290 Endometrial concentrations of metronidazole were very low after systemic treatment for 4 days.365
Mannose Infusion.
Development of resistance to multiple antibiotics has become an important issue in practice. The efficacy of specific sugar solutions in preventing bacterial adhesion to the endometrium has been investigated in vitro. Mannose solution significantly reduced adhesion of E. coli, P. aeruginosa, and S. equi subsp. zooepidemicus to the endometrium. Inhibition of adhesion of E. coli was possible with a concentration as low as 0.4 mg/mL, whereas inhibition of P. aeruginosa adhesion required a concentration of 75 mg/mL; a concentration of 3.13 to 25 mg/mL caused significant inhibition of S. equi subsp. zooepidemicus adhesion. N-acetyl-d-galactosamine inhibited adhesion of E. coli and P. aeruginosa only. If these results are confirmed in vivo, lavage with mannose solution might be a good adjunct or alternative to antibiotic therapy of endometritis.199
Plasma Infusion.
Intrauterine infusion of autogenous plasma (100 mL) anticoagulated with heparin or sodium citrate is suggested to enhance neutrophil function in endometritis-susceptible mares.14,18 Some clinical trials showed significant benefit with this approach,13,14,292 whereas others were unable to show enhanced bactericidal activity after plasma infusion.3,91,401 Infusion of plasma with leukocytes may improve pregnancy rate compared with infusion of plasma alone.257 The discrepancies between results may be caused by several factors, including the strain of bacteria and the amount and type of antibodies present in plasma. Infusion of serum from horses hyperimmunized against specific strains of S. equi subsp. zooepidemicus is reportedly more effective in increasing phagocytosis than the infusion of nonimmune serum.421
Prevention
Prevention of uterine infection is accomplished by decreasing the likelihood of contamination and by early recognition and treatment of PMIE. Contamination of the uterus can be prevented by correcting vulvar conformation and observing strict hygiene during breeding and genital manipulation. Susceptible mares should be monitored before breeding and bred using minimum-contamination breeding technique.195 The purpose of this monitoring is to reduce the chance of contamination of the uterus by bacteria and to eliminate the products of the inflammatory reaction caused by semen.
The primary mechanism for prevention of fluid accumulation in the uterus is contraction of the uterine musculature in response to oxytocin. Oxytocin release is observed in mares after mating, teasing, genital manipulation, and infusion of fluid into the uterine cavity.73,281,369 Oxytocin injection improves uterine defense by promoting uterine fluid evacuation.11,218,227,320 Administration of 20 IU of oxytocin intramuscularly (IM) induces uterine contraction for up to 90 minutes.246 Oxytocin administration to mares susceptible to PMIE at 4 hours after breeding aids in the elimination of excess fluid and improves fertility.25,320,336 The standard dose for an average mare is 10 IU intravenously (IV) or 20 to 25 IU IM. 70–72,246,320,392 Large doses may decrease uterine contractions.72,246 Oxytocin therapy may be repeated every 6 hours for 24 to 48 hours after breeding.278,392 A 7% increase in pregnancy rate is reported with a single injection of oxytocin (25 IU IM) within 72 hours of breeding.320 Oxytocin does not interfere with ovulation or gamete transport if administered no earlier than 4 hours after breeding.55,278
Prebreeding and postbreeding uterine lavage may be indicated in mares that tend to accumulate a substantial amount of fluid during estrus.57,59,60,407
Prostaglandin F2α (PGF2α; 5-10 mg IM) or its analog, cloprostenol (250 μg IM), is recommended for the treatment of mares with PMIE because of their ecbolic proprieties.55,220,278,396 Cloprostenol induces weaker but more sustained uterine contractions than oxytocin and is helpful when excessive uterine edema is present.93 Repeated administration of cloprostenol should be avoided because it has been associated with transient reduction of circulating progesterone levels during diestrus.55,278
Prophylactic in utero administration of antibiotics to PMIE-susceptible mare after breeding has been suggested. A combination of oxytocin treatment and intrauterine infusion of broad-spectrum antibiotics increases pregnancy rates in susceptible mares.4,320 However, such an approach may pose some risk of development of antimicrobial resistance.
Postpartum Metritis
Postpartum uterine infections are of particular importance because of their severity and effect on the general health of the mare. Septic postpartum metritis is often a result of nonhygienic manipulation during foaling, obstetric manipulations, and retained placenta. Mares with postpartum metritis may present with severe systemic complications of endotoxemia and laminitis. Treatment consisting of daily large-volume uterine flushes, systemic antimicrobial therapy, and appropriate therapy for endotoxemia and dehydration should be immediately initiated in affected mares.112,138,298
Other Infections of Nonpregnant Mares
Infectious vaginitis and cervicitis may occur as part of the uterine infection process or as a result of local irritation or laceration. Vaginal injuries secondary to breeding or parturition may lead to abscess formation and adhesions.33,101,261,322,343
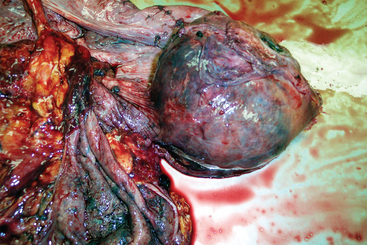
Infectious inflammation of the ovaries (oophoritis) with abscessation and peritoneal adhesions may occur after abdominal surgery or peritonitis (Fig. 8-5). Oophoritis may also occur as a consequence of repeated transvaginal ultrasound-guided follicular aspiration.47 Affected mares may present with abdominal pain, anorexia, fever of unknown origin, and weight loss. Transrectal ultrasonography may help in the diagnosis of these infections. Confirmation of the diagnosis and evaluation of the extent of the lesions may be achieved by laparoscopy. Ovariectomy is usually required for treatment of this condition.164,321
Salpingitis is rare in the mare but may result from ascending infection from the uterus after parturition.164 Salpingitis has been described in mares with contagious equine metritis (CEM)1 (see Chapter 41). Bilateral salpingitis results in sterility.
INFECTIOUS CAUSES OF ABORTION
Causes of equine abortion in several countries have been extensively reviewed (United Kingdom,304,359,431 United States,153,177,178,374–377 New Zealand and Australia,22 France,134,404 Egypt,101 India145). One third to half of all equine abortions are estimated to be the result of infection.359 Numerous organisms have been associated with infectious abortion, including viruses, bacteria, and fungi. The prevalence of each organism differs geographically. In England, equine herpes viral abortion predominates.359 In France, 79% of infectious abortions are caused by bacteria and 21% by viruses.134
Bacterial Abortions and Placentitis
Placentitis is a significant cause of equine late-term abortion, premature delivery, and neonatal death. It is implicated as a cause of abortion in as many as 50% of all mares that abort.* Placentitis is diagnosed in approximately 157 cases of fetal loss each year in Kentucky (approximately 30% of all submitted fetuses).445 It may be caused by a variety of bacterial, fungal, viral, and protozoal organisms.304,305,316 Bacterial placentitis is most common; fungal placentitis is reported in fewer than 10% of horses with placentitis.177,178,359
Placentitis is generally classified as three types: ascending, diffuse, and focal mucoid.445 With the exception of Leptospira spp., most bacterial or mycotic placentitis of mares is the result of an ascending infection.248,374
Ascendant Placentitis
Ascending placentitis is the most common type of placentitis in horses.306,316,445 Bacteria isolated from the placenta are comparable to those isolated from the uterus of mares with endometritis.175,331,445 Streptococcus equi subsp. zooepidemicus, E. coli, Pseudomonas aeruginosa, Enterobacter spp. and Klebsiella pneumoniae are the most frequent isolates.89,175,316,375,431
In a retrospective study of 954 cases of equine abortion, placentitis was recognized in 24.7% of all submissions.178 A bacterial or fungal organism was isolated in 68.6% of placentitis cases, and 57.4% of cases yielded bacteria both from the placenta and the fetal organs. The most common microorganisms isolated include S. equi subsp. zooepidemicus, E. coli, Leptospira spp., nocardioform Actinomyces, P. aeruginosa, Streptococcus equisimilis, Enterobacter agglomerans, K. pneumoniae, α–hemolytic streptococci, Staphylococcus aureus, and Actinobacillus spp.101,134 Other bacteria isolated include Proteus mirabilis, Citrobacter diversus, and fecal and environmental contaminants.178 Abortions resulting from hematogenous infection with Actinobacillus equuli425 and Corynebacterium pseudotuberculosis have been reported.145,308
There is a wide geographic variation in the frequency of specific bacterial and fungal isolates associated with equine placentitis and abortion. A high incidence of fungal placentitis is reported in England.431 The incidence of mycotic placentitis varies from region to region because of climate and other environmental factors.306,430 Emphysematous placentitis caused by clostridial infection was one of the top four causes of bacterial abortion in an early study.316 There are numerous reports of Enterobacter agglomerans placentitis in horses.* The importance of anaerobic bacteria, Mycoplasma spp., Chlamydia, and rickettsial organisms in equine placentitis is uncertain.108,319,335
Except for placentitis caused by Leptospira spp. or the nocardioform actinomycetes, most equine placentitis occurs in two forms: (1) acute diffuse placentitis with infiltration of neutrophils in the intervillous spaces or (2) focal necrosis of the chorionic villi. Placentitis from abortions before midgestation are chronic, focal, or focally extensive at the cervical area and characterized by necrosis of chorionic villi, presence of eosinophilic material on the chorion, and infiltration of mononuclear inflammatory cells in the intervillous spaces, stroma of villi, chorion, allantois, and vascular layer. Lesions may be either acute or chronic.178
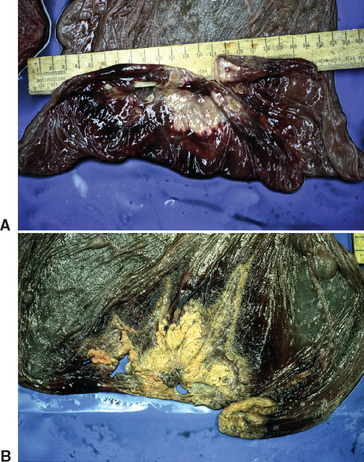
Bacterial placentitis most often induces abortion between 6 and 9 months of gestation. Placentitis resulting from E. coli tends to cause later abortion and more stillbirths. Placentitis from S. equi subsp. zooepidemicus tends to be acute and focal or diffuse. In acute bacterial placentitis the fetus is generally expelled before 8 months of pregnancy. Acute or diffuse placentitis may not be easy to recognize on gross examination of the placenta. Histologic evaluation of the allantochorion may reveal bacterial emboli with necrosis of chorionic villi or infiltration of neutrophils in the intervillous space. Chronic or focal placentitis typically results in birth of premature or weak foals or late-term abortions. Lesions tend to be located at the cervical star, where discoloration and thickening are observed (Fig. 8-6).
Escherichia coli placentitis is usually acute in mares that abort before 7 months of gestation but is more likely to be chronic and focally extensive, involving the cervical star, in mares that abort after 9 months of gestation. Placental edema and the presence of a white mucoid exudate on the chorion and fetal surface are common findings after abortion caused by E. coli placentitis (Fig. 8-7).
Gross and histologic features of mycotic placentitis were described in detail by Hong et al.178 Mycotic placentitis and abortion are most likely to occur in the late gestational period. Fungal organisms associated with equine abortion include Aspergillus spp.,121,145,177,303 mucoraceous fungi, Histoplasma caspulatum,153,345,373,374,445 Candida spp.,249,355,445 Mucor spp.,145 Coccidioides spp.,215,250,371 and Cryptococcus neoformans.34,300,341
Focally extensive placentitis is usually observed at the cervical star and adjacent area as a thick, leathery area. Histologically, except for histoplasmosis and candidiasis, the fungi induce a chronic, extensive placentitis characterized by extensive necrosis of the chorionic villi, neovascularization in the chorionic stroma, infiltration of neutrophils, mononuclear cells, or mixed inflammatory cells in the villi and chorionic stroma, and presence of fungal hyphae in the necrotic debris. Adenomatous hyperplasia with or without squamous metaplasia of the chorionic epithelium are frequently observed.178 Histoplasma capsulatum caused a multifocal granulomatous placentitis and abortion in one mare in the seventh month of gestation and three mares in the tenth month. Four newborn foals died from severe granulomatous pneumonia within a few days of birth, and a weanling Thoroughbred developed granulomatous pneumonia and lymphadenitis at 5 months of age.330
With Candida spp. infection, placentitis is generally diffuse, necrotizing, and proliferative with extracellular, yeastlike spores in the chorionic epithelium. Chronic, focally extensive placentitis is most common, with expulsion of foals late in gestation.178
Hematogenous Multifocal or Diffuse Placentitis
Multifocal or diffuse placentitis is less common than acute, focal placentitis and is usually a result of hematogenous spread of microorganisms to the uterus. This occurs with leptospirosis, salmonellosis, histoplasmosis, and candidiasis. A special focal mucoid form of placentitis, nocardioform placentitis, is emerging as common in several U.S. regions.178
Leptospira spp. Placentitis
Leptospira spp. placentitis is characterized by diffuse lesions secondary to hematogenous spread (see Chapter 34). Leptospirosis as a cause of placentitis seems to be more frequently diagnosed in Kentucky115,116,178,445 than in other regions of the world,411,431 probably because of specific regional characteristics and the difficulty in isolating or detecting the pathogen. An outbreak of leptospiral abortions has been described on a Thoroughbred farm in California after a flood.198
Most leptospiral abortions occur between 6 and 9 months of gestation. The affected placenta is thick, heavy, edematous, hemorrhagic, and occasionally covered with a brown mucoid material on the chorionic surface. Occasionally the affected placenta lacks detectable gross lesions. Green discoloration or cystic adenomatous hyperplasia of the allantois is observed in some cases. Fetal antibody against Leptospira spp. may be detected in foals by microagglutination test.178 Spirochetes are present in large number in the placental sections. Several species of leptospire have been isolated from aborted equine fetuses (e.g., L. pomona, L. grippotyphosa, L. interrogans serovars bratislava and pomona type kennewicki, serovar harjo type hardjoprajitno).* High-titer agglutinating antibody (>1:6400) may be observed in mares, but interpretation of serologic tests remain difficult without confirmation of infection by culture and isolation. Antibiotic treatment for 5 days (penicillin G) has been reported to help prevent abortion during an outbreak.198
Nocardioform Placentitis
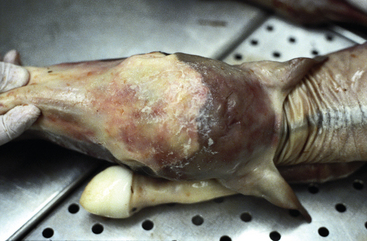
Nocardioform placentitis is a distinct type of equine placentitis first described in the United States in the late 1980s. Over the past 15 years an increasing number of cases of equine nocardioform placentitis have been diagnosed in Kentucky.117,153,177,445
Nocardioform actinomycetes induce a chronic placentitis that results in late-term abortion, stillbirth, or premature birth. The lesion is an extensive and severe exudative, mucopurulent, and necrotizing placentitis centered on the junction of the placental body and horns rather than the cervical pole.178 Infection of the placenta is generally thought to be a sequela of the hematogenous spread of the microorganisms from a primary port of entry.117,413 The fetus is often severely underdeveloped, with no remarkable gross or histologic lesions. The placental lesion is focally extensive (15-30 cm) and frequently located at the base of the uterine horns or at the junction between the body and horns of the placenta. The affected area is thickened, and its chorionic surface is covered with brown, necrotic, mucopurulent exudate and dotted with white or yellow granular structures. Underneath this mucoid material, the chorionic surface is reddish white, mottled, and roughened. Villous necrosis and adenomatous hyperplasia of the allantoic epithelium and hyperplasia with or without squamous metaplasia of the chorionic epithelium are frequently observed.78,178
Various groups of gram-positive, filamentous, branching bacteria have been implicated as etiologic agents in mares with nocardioform placentitis, including Nocardia spp., Rhodococcus rubropertinctus, and Amycolatopsis spp.49,124,177,413 However, most severe infections of this type are caused by the actinomycete Crossiella equi.118
A nocardioform isolate from equine placental lesions in the United States was determined by phylogenetic analysis to be closely related to Crossiella cryophila, a member of the genus Crossiella described in 2001.217 Subsequent polyphasic identification found that the isolates represent a new species of Crossiella, for which the name C. equi was proposed.118 Polyphasic taxonomic studies on actinomycete strains isolated from equine placentas from horses in Kentucky and southern United States indicate that the isolates are members of the genus Amycolatopsis. It is proposed that these strains be classified as three novel species of the genus Amycolatopsis and named Amycolatopsis kentuckyensis, Amycolatopsis lexingtonensis, and Amycolatopsis pretoriensis.213
During the 2002 and 2003 foaling seasons, Cellulosimicrobium (Cellumonas) cellulans (formerly Oerskovia xanthineolytica) was isolated from fetal tissues or placentas from cases of equine abortion, premature birth, and term pregnancies in Kentucky. Significant pathologic findings included chronic placentitis and pyogranulomatous pneumonia. In addition, microscopic and macroscopic alterations in the allantochorion from four of seven cases of placentitis were similar to those caused by Crossiella equi and other nocardioform bacteria.48
Pathogenesis and Diagnosis of Placentitis
Because of the importance of placentitis in the pathogenesis of bacterial abortion, researchers at the University of Florida developed models for study of ascendant placentitis to gain insight into the pathogenesis of disease and provide a method to optimize diagnostic and treatment recommendations.225,226,244,262,368 Bacterial infection of the chorioallantois induces an increase in expression of proinflammatory cytokines (IL-6 and IL-8) in placental tissue. Subsequent release of PGE2 and PGF2α into the allantoic fluid leads to premature delivery.223,226,262 The premature delivery of the fetus is most likely caused by acceleration of the fetal maturation process induced by changes in placental function. The resulting endocrine changes lead to increased uterine contractures and intrauterine pressure, causing dilation and induction of labor. A premature increase in maternal plasma progestins may be an indication of accelerated fetal maturation or fetal stress. Foals may survive if they are near term (>305 days).225,286,287
Clinically, placentitis is suspected in mares with premature udder development or lactation and vaginal discharge. However, most mares with placentitis do not show any outward signs of infection.225,445
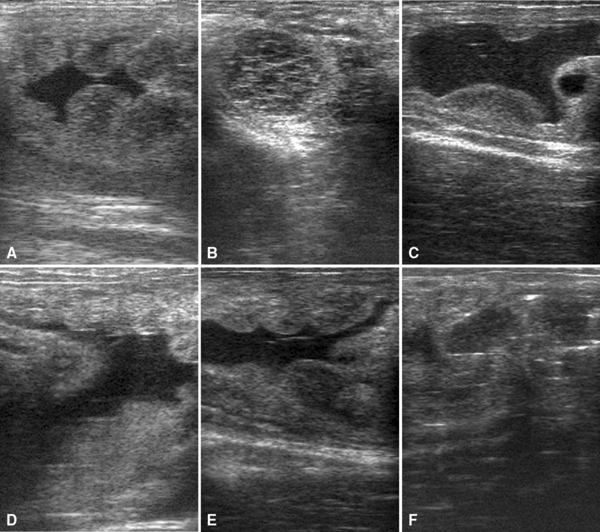
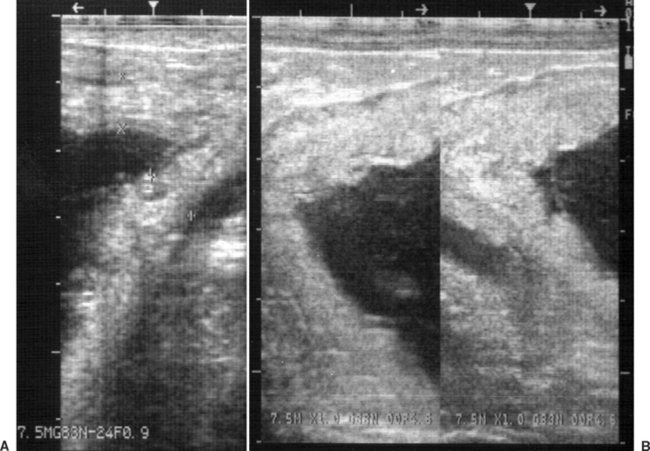
Placentitis may be diagnosed by transrectal and transabdominal ultrasound examination.66,402,445 Measurement of the combined thickness of the uterus and placenta (CTUP) by transrectal ultrasonography is particularly helpful in the diagnosis and monitoring of ascendant placentitis.193, 327–329,390,394,400 The measurements are obtained 2.5 to 5 cm cranial to the cervical-placental junction using a 5- or 7.5-mHz linear transducer. The area measured should be on the ventral aspect of the uterine body just above the middle branch of the uterine artery (Fig. 8-8). Normal CTUP for light horses is less than 8 mm between 271 and 300 days of gestation, less than 10 mm between 301 and 330 days of gestation, and less than 12 mm from 330 days of gestation to term.26,193,327,328,394 These measurements are slightly higher in Warmblood and Draft horses and lower in ponies.26,66,193 Placental malfunction has been associated with CTUP of greater than 15 mm in horses and greater than 12 mm in ponies after 310 days of gestation.66,225
During ultrasonographic evaluation, other features of infectious placentitis may be identified. These include placental separation and accumulation of purulent hyperechoic heterogenous fluid between the endometrium and the placenta and increased echogenicity of fetal fluid. Increased echogenicity of fetal fluid is caused by meconium, inflammatory debris, and hemorrhage.225
Endocrinologic evaluation may also help in determining placental pathology and risk for abortion. The most important hormones evaluated are progestins, which are relatively stable during a normal pregnancy. A change in serum progestin concentration (increase or decrease) by more than 50% or a value that is constantly out of reference laboratory range signals placental pathology or fetal stress.225,288
After abortion or premature birth, most cases of chronic placentitis are easily recognized on gross examination, but microscopic histologic examination is important to determine the presence of acute placentitis359 (Fig. 8-9). In acute placentitis the infection may be contained within the placenta, and the fetus is usually sterile. Some foals may be born alive with neonatal septicemia.359
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree