CHAPTER 83 Reproductive Biotechnologies in the Goat
In recent decades, scientists have contributed to develop powerful biotechnologies such as estrus synchronization, superovulation, artificial insemination, embryo collection and transfer, in vitro embryo production, cryopreservation, a variety of embryo micromanipulation procedures, and nuclear transfer. The development of these technologies enables the manipulation of animal reproduction to rapidly propagate superior or desired animals and genes, the genetic modification of animals to improve their production quality, and the production of medically significant products. The goat’s diversified commercial value, size, relatively short gestation period (when compared to the cow), and popular demand make it a convenient species for new technologic research and application.
EMBRYO COLLECTION AND TRANSFER
Synchronization and Superovulation
Many combinations of treatments for the purposes of embryo collection and transfer are available. Estrus can be synchronized by the administration of progestogens such as progesterone implants or synthetic progestins (flurogestone acetate, FGA; medroxyprogesterone acetate, MAP) given either orally or by the insertion of a vaginal sponge. The most widely used synchronization device for goats is the control internal drug release progesterone implant (CIDR-G, 0.3 g progesterone, Eazi Breed, InterAg, New Zealand) which is inserted in the goat’s vagina using a special applicator. Most traditional schemes consist of a long progestagen (12–18 days) treatment; recent protocols use a shorter progestagen treatment (5–9 days) accompanied by a prostaglandin F2 alpha (PGF2α) analogue injection.1 During the breeding season an analogue of PGF2α is commonly given at the time of device insertion to destroy the corpus luteum and obtain a greater percentage of estrus synchronization. The method for synchronization of estrus is the same for both the donor and the recipients. Owing to the hormonal treatment administered to donors, estrus occurs sooner than in the recipients; therefore implants should be removed from recipients 12 hours prior to removal of the progesterone or progestin source from donors. Some synchronization programs recommend the replacement of the progesterone or progestin source midway through a long synchronization regimen to maintain high levels of progesterone during gonadotropin therapy.
For the induction of superovulation of donor goats, pituitary extracts of follicle-stimulating hormone (FSH) and pregnant mares’ serum gonadotropin (PMSG; also called equine chorionic gonadotropin, eCG) are the gonadotropins most used. Commercially available FSH products are: Folltropin V (Vetrepharm, Ontario, Canada), Ovagen (ICP, Auckland, NZ) and Super-Ov, (Ausa International, Tyler, TX). Several protocols can be used for superovulating goats, most commonly the injection of multiple doses of FSH on the last 3 to 4 days of the progestagen treatment. Due to the short half-life of the FSH molecule, it is traditionally administered every 12 hours. One example is the twice-a-day injection of a series of decreasing doses of FSH (5, 5; 3, 3; and 2, 2 mg per injection), with a total dose of 20 mg, with the next to last injection accompanied by progesterone removal and an injection of 150 μg of a PGF2α analogue. Goats display behavioral estrus at approximately 24 hours after implant removal. Folltropin V can also be administered in decreasing doses (3.6, 3.6; 3.2, 3.2; 2.4, 2.4; and 1.6 mg) with a 17-day progestagen treatment; a simpler protocol consists of six injections at a level dose given every 12 hours (total dose equivalent to 20 mg NIH-FSH-P1).2 An average of 17 ovulations have been obtained per doe using this protocol.3 Super-Ov is a highly purified preparation of FSH that is marketed in units of FSH activity per milliliter rather than in milligrams per milliliter. Doses are calculated as milliliters of the reconstituted product; a 10-ml vial contains 75 units of activity. One commercially used regimen of Super-Ov in goats is a 4-day declining dose (1.0, 1.0; 0.8, 0.8; 0.6, 0.6; and 0.4, 0.4 ml) that is reduced by 0.2 ml per injection for young does. Another example is a level dose of 0.7 ml bid for 4 days. Superovulatory treatments using Ovagen (which has a consistent level of LH activity) consist of eight IM injections of 1.25 ml given twice daily starting 60 hours before progestagen removal. The response to this protocol has been reported to be of 14.3 ± 0.5 corpora lutea, 11.3 ± 0.5 recovered embryos, and 6.8 ± 0.4 viable embryos per goat.4
Recently the use of estrogens at the time of progesterone implant insertion and removal have improved superovulation responses. The ovarian response in terms of number of corpora lutea has been shown to be correlated to the number of dominant follicles at the onset of gonadotropin treatment. A higher number of 2- to 6-mm follicles tended to be associated with a lower embryo recovery rate. Estrogens (such as estradiol benzoate, EB) have been shown in the bovine to induce regression of those dominant follicles, which are likely to be immature or in the process of atresia, followed by a synchronous follicular wave emergence (4–5 days after EB injection). This wave generally coincides with the time of the administration of the FSH treatment. Therefore, at the time of FSH injections a uniform cohort of follicles is recruited and no dominant follicles are present.2,4,5
When PMSG is used, a single injection 24 to 48 hours prior to implant removal is administered to the donor doe. Doses of PMSG range from 500 IU to 1500 IU, depending on breed, age, and size of doe and previous responses to treatment. One of the disadvantages of using PMSG is the tendency to produce persistent follicles that interfere with fertilization and overall embryo collection rates.6
Estrus Detection and Breeding
Estrus is detected in donor does by observation of mating; in recipients estrus should be detected using vasectomized bucks or androgenized females. The time from beginning of estrus to ovulation in goats is approximately 32 to 36 hours. If laparoscopic artificial insemination is to be performed, does should be inseminated 12 and 24 hours after the animals were first seen in estrus or 45 to 50 hours after implant withdrawal.7
LAPAROSCOPIC INSEMINATION
Laparoscopic artificial insemination allows semen to be placed directly into the lumen of the uterine horns considerably closer to the site of ovum fertilization. High fertilization rates (80%)8 can be achieved with this technique, compared with transcervical insemination (50%) using fresh diluted semen.7
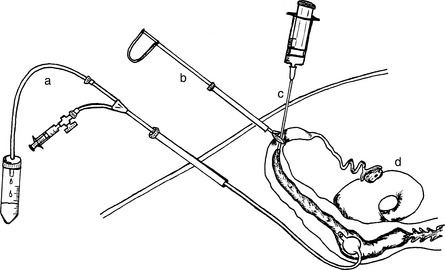
The goat is secured in a laparoscopic cradle and is tilted head down at an angle of 40 degrees or more. Pneumoperitoneum is produced by introducing CO2 through a Verres needle inserted transabdominally. This separates the abdominal wall from the viscera, clearing the field for manipulation of the reproductive tract. The trocar and cannula for the laparoscope (5–7 mm diameter) are inserted into the peritoneal cavity approximately 10 cm cranial to the udder and to the left of the midline. The secondary trocar and cannula (5 mm diameter) are inserted to the right of the midline. The laparoscope is inserted through the cannula and the uterus is located just cranial to the bladder. An assistant prepares the inseminating gun (IMV International Corp-USA, Minneapolis, MN) or pipette (glass) and hands it to the operator. The operator guides the tip of the inseminating gun toward one uterine horn. Care should be taken not to perforate any viscera with the sharp needle or tip of the inseminating device. While visualizing through the laparoscope, the site of insemination is selected along the major curvature of the uterine horn and the needle is stabbed through the myometrium and into the lumen in a perpendicular position (Fig. 83-1). Semen should be deposited with no resistance and no swelling of the uterine wall should be seen, indicating that the needle is correctly positioned in the lumen of the uterine horn. The inseminating gun is removed, and the operation is repeated on the other uterine horn. The instruments are removed and the abdominal wounds are sutured. In most cases the dose of semen contained in one straw (40–100 million motile sperm cells) is divided in both uterine horns. If fresh semen is being used, reports indicate that the effective dose is about 20 × 106 for laparoscopic AI, and 100 × 106 for cervical AI.8
Anesthesia: Animal and Equipment Preparation
Animals are taken off feed 18 to 24 hours prior to any surgical procedure. The animals are anesthetized with an anesthetic of choice. A review of commonly used anesthetic regimens is available.9 In this author’s laboratory, a combination of xylazine and ketamine (0.15 mg/kg xylazine and 3.6 mg/kg ketamine) is used intravenously to perform laparoscopic embryo transfer into recipients or for induction when gas anesthesia is used for embryo collection. When gas anesthesia is not available, a combination of xylazine and ketamine can be used alone (0.22 mg/kg xylazine and 5.5 mg/kg ketamine), with half the dose administered IV and half IM to maintain anesthesia during embryo collection. If necessary, animals can be re-dosed with 1.9 mg/kg ketamine IV. Because of profound effects of higher doses of xylazine (>0.15 mg/kg) on cardiovascular and respiratory parameters in sheep, it has been recommended that dosing in goats also be as conservative as possible.9 The effects of xylazine can be reduced postoperatively by administration of an antagonist such as yohimbine (0.11 mg/kg IV). However, if ketamine levels are still elevated at this time, reversal of xylazine will result in tremors. A combination of tiletamine and zolazepam (Telazol, Fort Dodge Laboratories, Fort Dodge, IA) at a dosage of 5.5 mg/kg IV has been reported to provide at least 1 hour of anesthesia.9 In our experience, when goats are elevated in a laparoscopic cradle, a second dose of anesthetic is frequently needed within 30 minutes. In these cases, doses of 0.5 to 1 mg/kg are administered IV as needed.
Embryo Collection
The first successful embryo collection and transfer in goats was reported by Warwick and associates.10 Although their interest was in creating hybrids between goats and sheep, they reported the birth of a kid to a doe following surgical collection of a four-cell goat embryo and replacement of the embryo in the donor’s reproductive tract. It was not until 30 years later that further reports on embryo transfer in goats appeared.11
In most instances, surgical procedures have been used for the recovery of embryos from goats. However, alternative techniques for collecting embryos from goats, such as laparoscopic and nonsurgical or transcervical embryo collection techniques, have been developed. The embryo collection technique to be used depends on the day that the embryos are to be collected. Embryos can be collected as early as day 1 after breeding, or as late as day 7 or 8 after breeding.6,12–15
Early luteal regression (ELR) has been reported in synchronized and superovulated goats.16,17 Early luteal regression is characterized by small, white-pink corpora lutea and may occur in 10 to 32% of treated animals by the time of embryo collection (days 5–7 after breeding) or transfer. Embryo recovery rates from donor does with ELR are poor; there are observations that ELR can be reduced by administering a luteolysin inhibitor during the period between breeding and embryo collection. Also, progestins or progesterone administered during this period may help reduce the effect of ELR by keeping progesterone levels high during the early stages of embryo development (days 0–7). Another way to avoid embryo loss associated with ELR is to collect embryos on day 3 after breeding, before luteal regression occurs and embryos are expelled from the reproductive tract. Embryos are located in the oviducts at this stage; therefore, an oviductal flush is required.
Surgical Embryo Collection
Although there has been some progress in developing alternative methods for collecting embryos from small ruminants, goat embryos are most commonly harvested by surgical means.14 This ensures high recovery rates, which can reach 100%. Animals are positioned in dorsal recumbency position with an inclination of 20 to 30 degrees with the head down. Three procedures involving laparotomy have been described for the collection of embryos, all of which involve exteriorization of the uterus through a midventral incision with or without examination of the ovaries to determine the response to superovulation treatment. This procedure should be carried out by skilled surgeons with attention to the principles of surgery; otherwise adhesions of the reproductive tract will develop.
In the first procedure, embryo flushing medium is injected into the uterine horn and expressed out through a fluted plastic catheter inserted into the oviduct through the fimbriated end. This technique can be used for collecting oviductal-stage embryos.14 In the second procedure, the oviductal contents are flushed from the fimbria into the uterus and out of the uterus through a Foley catheter (size 8 Fr) inserted in the uterine horn near the bifurcation.18 This technique can be used for collecting embryos at any stage. In the third procedure, the uterine horn is flushed from the tip near the uterotubal junction toward a Foley catheter (8 Fr) inserted in the uterine horn near the bifurcation.12,19,20 This technique results in fewer adhesions because it eliminates the need to manipulate the oviduct, fimbria, and ovaries. However, the technique is limited to use 4 or more days after estrus, at which time the embryos will have reached the uterus.21 Embryo collection rates using the surgical approach in goats average 85%.12,20
Although anecdotal reports of numerous repeated surgical collections from individual goats exist, several other reports have shown detrimental effects of repeated surgical embryo collections. These effects may be noticed as soon as the second embryo collection is performed in the form of adhesions to the reproductive tract if inadequate surgical procedures are followed during embryo collection.22 This is a potential limiting factor for the use of embryo transfer for genetic improvement. Therefore, it is important that the surgeon minimize handling the reproductive tract, and once the uterus or part of the uterus is exteriorized, it should be continuously moistened using a soft mist of saline solution and antibiotics.
Laparoscopic Embryo Collection
Laparoscopy has been a useful tool in reproduction for small ruminants. Several researchers have applied this technique to investigate the role of ovarian function in the control of the reproductive cycle,23,24 to determine the time of ovulation,25,26 to perform artificial insemination,27 and to collect and transfer embryos.12,28,29 It has been reported that in goats the number of ova and embryos collected by laparoscopy compared with the number of corpora lutea (CL) (recovery rate) is approximately 15% lower than through surgery.12 On the other hand, laparoscopic embryo collection can be repeated successfully on the same goat with few complications. It eliminates the need for exteriorization and manipulation of the reproductive tract and for suturing of the body wall. This prevents formation of postoperative adhesions. However, as mentioned earlier, the same surgical care should be emphasized during these procedures.
As an early alternative to laparotomy for the collection of uterine-stage embryos from sheep, a laparoscope was used to visualize the ovarian response and to exteriorize the tip of one uterine horn at a time through a small incision.30 Flushing medium was introduced into the tips of the horns through an intravenous catheter and was collected through a glass vaginal speculum placed against the external os of the cervix into a glass recovery dish. This procedure yielded a 46% embryo recovery rate.
Very little research has been reported on the use of laparoscopic embryo collection and transfer in goats.12,28,29 However, a great deal of laparoscopic embryo transfer research has been conducted in sheep31 and these techniques are applicable in goats.32 The donor animal is positioned in the laparoscopic cradle, and pneumoperitoneum is created. The primary trocar and cannula are inserted into the peritoneal cavity approximately 10 cm cranial to the udder and 2 to 3 cm to the left of the midline. Care should be taken to avoid major vessels under the skin and internal organs. The secondary trocar and cannula (5 mm diameter) are inserted to the right of the midline. The laparoscope (5–7 mm diameter) is inserted through the primary cannula and the uterus is located just cranial to the bladder. In some cases, the omentum or visceral fat may obscure the uterus. These can be pushed cranially with a manipulating rod placed through the secondary cannula. Once the uterus is located and the ovulation points counted and evaluated, a third cannula (5 mm in diameter) is inserted on the midline 2 cm cranial to the others. Through this third cannula, a long blunted paravertebral needle (12 gauge, 25 cm long) is used to make a puncture wound in the uterine wall cranial to the bifurcation of the uterus, which is secured through the second cannula with atraumatic laparoscopic forceps. This allows the insertion of a balloon Foley catheter with stylet through the small puncture into the uterine lumen. The balloon on the catheter is inflated and the stylet removed. The uterus is secured by the inflated balloon.
If a three-way catheter is used, atraumatic grasping forceps must be used to block the uterotubal junction to prevent flow of the medium and embryos toward the oviduct. The flushing medium is introduced through the catheter and into the uterine horn by means of an inner extendable tube that reaches the uterotubal junction. Return of the flushing medium is initiated by pressure and gravity. Each horn is flushed with 30 ml of medium, and 79% embryo recovery rates have been achieved.12
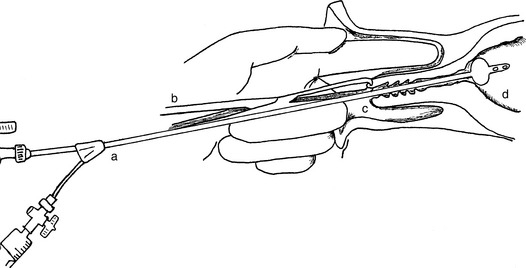
If a two-way catheter is used (Fig. 83-2), the tip of the uterine horn is secured with atraumatic forceps and cannulated using an intravenous catheter (18 gauge, 5½ inches long). After the stylet is removed, a collection medium (60 ml) is introduced through the intravenous catheter and collected through the Foley catheter. The procedure is repeated for the other horn, the instruments are removed, and the abdominal incisions are closed. With this technique collection rates range from 50% to 80%.29,31
Nonsurgical (Transcervical) Embryo Collection
Nonsurgical (transcervical) embryo collection techniques avoid the formation of postsurgical adhesions and maintain the value of genetically superior donors following multiple embryo collections. Moore stated that nonsurgical embryo collection procedures, as used in the cow, would appear difficult owing to the narrow and tortuous nature of the cervical canal of ewes and does.33
Nevertheless, reports of successful nonsurgical embryo collection in goats have been published.34–37 These groups obtained embryos at the blastocyst stage by priming the cervix with a combination of prostaglandins E2 and estradiol,38 dilating the cervix prior to flushing with a Laminaria japonica tent or mechanically using an oral probe designed for use in rats. The tents were inserted into the cervical canal for 6 to 12 hours and held in place by packing of the cranial vagina with gauze. The flushing procedures were performed in standing animals with a variety of catheters such as a modified two-way 24-gauge Foley catheter through which a stainless steel tube (50 cm long, 1.5 mm inner diameter) was inserted. A Sovereign catheter (5 Fr) was inserted through the stainless steel tube to introduce the medium. Other flushing cannulas were based on the model of the two-way catheter that was developed for use in cattle.35 The cannula was a concentric double tube with a metallic inner tube (Cathelin needle) for inflow of flushing medium and a polyethylene outer tube for outflow. Using this catheter, the practitioner flushed each uterine horn individually by pulling the catheter back into the uterine body prior to reinsertion into the other horn. Some of the problems encountered during these procedures were the incomplete passage of the cannula through the cervical lumen and the puncture of the uterine wall with the flushing device. Embryo recovery rates were 89.5% for 15 females (34/38 CL), but the collection rate among all the subject animals was not very high.
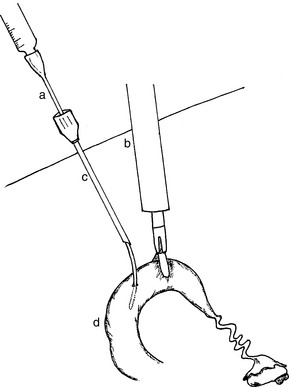
A method for catheterizing the sheep cervix and collecting embryos nonsurgically without the aid of a cervical dilator was developed by Coonrod and associates.39 This procedure has been adapted for use in goats.29,40,41 Animals are tranquilized or anesthetized and positioned in dorsal recumbency. The cervix is visualized using a vaginal speculum and a strong light. A section of the external cervical os is grasped with long Allis tissue forceps. The forceps and the cervix are retracted into the vagina. A catheter (e.g., Verres needle, section of urinary catheter size 8 or 10 Fr with stylet, Ott catheter size 8 Fr) is placed into the external os of the cervix as far in as possible. The vaginal speculum is removed and the index finger of a gloved hand is inserted into the vagina alongside the retracted cervix. The finger is used to guide the catheter through the cervix. Once the catheter is in the body of the uterus or in one of the horns, the stylet is removed and the balloon filled (when catheters with balloon are used) (Fig. 83-3). The tubing for inflow and outflow is connected to the catheter by means of a three-way stopcock, and small amounts of medium (5–10 ml) are introduced at each flush to keep the pressure low and minimize fluid leakage. The catheters without balloons are moved slightly in and out to induce return of fluid. Massaging the abdominal area toward the pelvic cavity helps to recover the flushing medium. The return medium is collected in an embryo filter via the outflow tubing. A novel approach for improving the practicability of the nonsurgical method for embryo collection in goats is the administration of a luteolytic dose of PGF2α 8, 16, or 24 hours before flushing. This procedure will decrease the plasma progesterone concentration and luteolysis and will promote uterine contractility in response to infusion of the flushing medium, increasing from 43% to 80% embryo recovery rates.36,37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree