A ruptured bladder is the most common cause of uroperitoneum in the horse, and initial clinical signs include depression, anorexia, and colic (Tuohy et al. 2009). The fluid shift from the bladder to the peritoneal cavity results in progressive abdominal distention (Walesby et al. 2002; Tuohy et al. 2009) and uremia (May et al. 2008). Because the osmolality of urine is two to three times greater than that of interstitial fluid, water leaves the blood and interstitial compartment, and then enters the abdominal cavity, which results in hypovolemia and dehydration (May et al. 2008).
Additional clinical signs of uroperitoneum may include intestinal ileus, a reduced passage of feces, and pollakiuria (Walesby et al. 2002; Tuohy et al. 2009). Increased intra-abdominal pressure can lead to compromised cardiopulmonary function, exhibited by tachycardia, tachypnea, a weak pulse, and increased capillary refill time (Tuohy et al. 2009). Clinical signs may be delayed for 3–5 days in both neonates and adult horses (Table 21.1) (Walesby et al. 2002).
Table 21.1 Clinical signs of uroperitoneum
| Clinical Signs of Uroperitoneum | |
| Clinical Signs | Pathophysiology |
| Depression | |
| Anorexia | |
| Colic | |
| Progressive abdominal distension | Fluid shift from the bladder to the peritoneal cavity |
| Hypovolemia/dehydration | Water follows the osmotic gradient (established by the urine) from the blood into the abdominal cavity. |
| Intestinal ileus | Dehydration |
| Reduced passage of feces | |
| Pollakiuria | Inability of the bladder to retain fluids |
| Tachycardia, tachypnea, weak pulse, increased capillary refill time (CRT) | Increased intra-abdominal pressure leading to compromised cardiopulmonary function |
Definitive diagnosis of uroperitoneum and a ruptured bladder can be accomplished primarily by two techniques. The first technique is achieved by conducting a peritoneal fluid analysis in conjunction with a serum biochemistry panel (Walesby et al. 2002; Tuohy et al. 2009). A peritoneal creatinine concentration twice that of plasma is considered to be diagnostic for uroperitoneum (May et al. 2008; Tuohy et al. 2009). Urea is small and readily diffuses across membranes, whereas creatinine molecules are large and diffuse poorly across the peritoneal membrane, making creatinine a valuable diagnostic tool (Walesby et al. 2002; May et al. 2008). The identification of calcium carbonate crystals in the peritoneal fluid is also considered diagnostic for uroperitoneum (May et al. 2008). Biochemistry panel abnormalities associated with uroperitoneum include azotemia, hyperkalemia, hyponatremia, and hypochloremia (Walesby et al. 2002; Tuohy et al. 2009). Relative to plasma, sodium and chloride ion concentrations are low in urine, and potassium ion concentrations are high in urine (Walesby et al. 2002; May et al. 2008). The resulting ion shifts of uroperitoneum result in hyperkalemia, hyponatremia, and hypochloremia (Walesby et al. 2002; May et al. 2008). As the urinary bladder becomes distended beyond capacity, ureteral and renal tubular pressures increase, causing a reduction in glomerular filtration rate and renal blood flow (May et al. 2008). This results in azotemia and decreased elimination of creatinine, blood urea nitrogen, and other metabolic waste products, which may lead to metabolic acidosis, hyperphosphatemia, and hypercalcemia (Table 21.2) (May et al. 2008).
Table 21.2 Biochemical abnormalities
| Biochemical Abnormalities | |
| Abnormality | Pathophysiology |
| Azotemia | Bladder distension causes increased ureteral and renal tubular pressures, and reduces glomerular filtration rate and renal blood flow. |
| Metabolic waste | |
| Hyperphosphatemia | |
| Hypercalcemia | |
| Hyperkalemia | Potassium concentrations are high in urine and low in plasma normally: The osmotic shift from the uroperitoneum drives potassium into blood. |
| Hyponatremia | Sodium and chloride concentrations are low in urine and high in plasma normally: The osmotic shift from the uroperitoneum drives sodium and chloride into the peritoneal cavity. |
| Hypochloremia | |
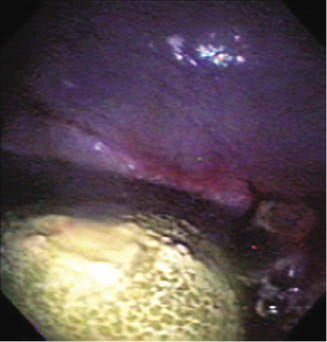
The second diagnostic technique is endoscopic examination (Figure 21.2) with a transabdominal ultrasound (Walesby et al. 2002; Tuohy et al. 2009). In a patient with uroperitoneum, a transabdominal ultrasound will reveal a large quantity of anechoic fluid free within the abdominal cavity (Walesby et al. 2002). Endoscopy with a flexible fiber-optic endoscope introduced through the urethra is used to evaluate the integrity of the urinary bladder and has proved to be particularly useful in providing direct viewing of bladder ruptures (Figure 21.3) (Walesby et al. 2002).
Rationale for the Procedure
Bladder rupture is very painful for the patient and potentially life threatening. Peritonitis, more commonly reported in adult horses, frequently develops following intraperitoneal rupture of the urinary bladder (Walesby et al. 2002). Urine is irritating to the peritoneal cavity and can predispose the patient to bacterial infection (Walesby et al. 2002).
Horses suffering from uroperitoneum will often present with a history of dribbling urine, episodes of colic, transrectal palpation of the thickened urinary bladder, and abdominocentesis with a large amount of fluid (Tuohy et al. 2009). The horse is often reluctant to walk, stands hunched over, and develops severe abdominal distention (Walesby et al. 2002). It is critically important that lesions be diagnosed early if the urethra is occluded by a squamous cell carcinoma because progression of neoplasia may result in metastasis to the lymph nodes, mesentery, abdominal organs, or lungs (May et al. 2008).
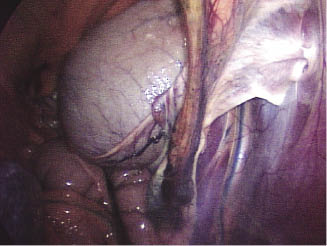
Nonsurgical methods of treating uroperitoneum have been utilized successfully by removing urine via urinary catheters and abdominal drains (Figure 21.4) (May et al. 2008; Tuohy et al. 2009). The urinary bladder displays a remarkable capacity for healing and is able to regain approximately 100% of prewounding strength in 14–21 days, and complete re-epithelialization can occur in 30 days (May et al. 2008). A combination of bladder decompression with urine drainage from the abdomen may allow the bladder wall to spontaneously heal. Additional complications such as peritonitis may slow or obstruct the healing process (Tuohy et al. 2009). The size and location of the rupture can play a major role in determining whether nonsurgical management of the problem will be successful (Tuohy et al. 2009). To date, there does not appear to be a prescribed limit for the size of the tear or defect that would accommodate nonsurgical management, but a 5-cm defect is one of the larger ones reported to have been managed conservatively (Tuohy et al. 2009).
Figure 21.4 Photograph showing abdominal drains in a foal with a ruptured bladder.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree