Introduction
The primary goal of any rehabilitation treatment program is to maximize each patient’s functional recovery. This may be achieved through a variety of methods including therapeutic exercises, manual techniques, functional mobility retraining, and use of assistive devices. Several physical modalities are available to augment and complement the treatment plan. Their use rarely comprises the bulk of a treatment plan. Used with an understanding of the effects each has on different tissues, these tools can be effective in reducing pain, facilitating muscle strengthening, improving flexibility, and supporting tissue healing, thereby maximizing the patient’s ability to participate in and benefit from other aspects of the treatment plan.
The decision to use any physical modality must be based on a thorough assessment of the patient’s status. Frequent reassessment will also help to progress the use of a modality, transition between modalities, or discontinue use altogether at the appropriate time.
This chapter presents a general overview of the physical modalities most commonly used in canine rehabilitation. Each section reviews the basic mechanisms of action, some of the evidence supporting its use, and recommendations regarding clinical application of each modality.
The scope of this chapter does not permit a detailed explanation of the physics of each modality, nor does it provide a comprehensive list of the research related to each. Textbooks dedicated to physical modality use in rehabilitation medicine are available and should be referenced for additional information (Michlovitz & Nolan, 2005; Cameron, 2008).
Superficial Thermal Agents: Cold and Heat
Superficial thermal agents are used to decrease pain, reduce swelling, promote healing, and improve flexibility. They are often the most convenient modalities as they are readily available, involve minimal expense, and are frequently safe to use as part of a home treatment program.
Superficial Cold: Cryotherapy
Cryotherapy is the use of cold to remove energy from tissues in the form of heat. Cold application can provide tissue cooling 2–4 cm deep (Nadler et al., 2004).
Evidence Supporting Cryotherapy Use
The initial physiologic response to cryotherapy is cutaneous blood vessel vasoconstriction (Olson & Stravino, 1972). A resultant reduction in blood flow to the area occurs (Weston et al., 1994). This can be useful when treating superficially located muscles, tendons (Knobloch et al., 2007), and joints (Cobbold & Lewis, 1956). Of note, cooling tissues for an extended time, or to significantly lower temperatures (less than 10°C), can result in cycles of arteriolar vasodilation, often referred to as the hunting response. This may be avoided by cycling cold application (e.g., 20 minutes on, 10 minutes off, 10 minutes on) (Karunakara et al., 1999). When treating injured tissues, some evidence has been found that cryotherapy use for at least 20 minutes can lead to dilation of local venules, which may additionally contribute to edema reduction (Smith et al., 1993). Regardless of the mechanism, a reduction in swelling has been demonstrated when cryotherapy is used after tissue trauma.
Cold application after injury can reduce tissue metabolism (Albrecht et al., 1996). This decreases cellular oxygen demands and may diminish secondary hypoxic cellular injury. A 2006 study demonstrated this with three 20-minute cryotherapy sessions immediately following trauma to muscle tissue (Oliveira et al., 2006).
When considering the effects of cryotherapy on peripheral nerves, sufficiently lowering temperatures can decrease both sensory and motor nerve conduction velocities (Greathouse et al., 1989; Dioszeghy & Stalberg, 1992). Caution should be exercised, however, when applying cryotherapy over superficially located nerves as neuropraxia or axonotmesis is a risk (Bassett et al., 1992; Moeller et al., 1997).
Frequently, a primary goal with cryotherapy is pain reduction. This may occur through the decreased nerve conduction velocity mentioned above. Analgesia may also result via the gate control theory of pain with overstimulation of cold receptors blocking the transmission of pain signals to higher centers. Furthermore, a 2007 study demonstrated that cold application can increase both pain threshold and pain tolerance (Algafly & George, 2007). Studies have shown that human patients who receive cryotherapy following orthopedic surgery often require less analgesic medication (Ohkoshi et al., 1999).
Cold has been shown to temporarily reduce the spasticity often seen with central nervous system lesions. A number of studies (Knutsson, 1970; Allison & Abraham, 2001) have demonstrated this with a resultant greater ease of movement.
Cryotherapy can be used to reduce muscle spasm. This may be related to a decreased firing rate of muscle spindle and Golgi tendon organ receptors (Mense, 1978). A reduction in EMG activity following cryotherapy has been demonstrated, thought to indicate a reduction in muscle spasm (Prentice, 1982).
Finally, short periods of cryotherapy may increase muscle force production, possibly due to increased muscle blood flow or motor nerve activity (McGown, 1967; Knutsson & Mattsson, 1969). This may be useful to stimulate activity of weak muscles. Longer periods of cold application, however, can decrease muscle strength (Johnson & Leider, 1977).
Considerations for Clinical Application of Cryotherapy
Most commonly, cryotherapy is provided with cold packs and ice massage. When determining which method to use, the size and accessibility of the treatment area should be considered as well as the patient’s tolerance. Cold packs can include gel packs, crushed ice packs, and water-alcohol slushes. Gel packs can be purchased in various sizes to fit different body parts, and they often include a sleeve to protect the skin (Figure 7.1). Cold packs designed to fit specific parts on the dog are also available (Figure 7.2).
Figure 7.1 Various types of cold packs, including different sized sleeves to wrap different anatomical sites.

Figure 7.2 Cold packs can be purchased that are fitted to specific anatomical structures.
Image courtesy of CanineIcer.com.

Cold packs can be made in the clinic with crushed ice or by freezing a combination of three parts water to one part alcohol in a sealable bag. A thin damp towel should be placed between the pack and the patient unless the hair coat is thick. The depth of target tissue, duration of cold application (Figure 7.3), ability of surrounding tissues to conduct heat (Bierman & Friedlander, 1940), and temperature of the agent compared to the target tissue temperature should be considered.
Figure 7.3 Temperature changes at different tissue depths during cryotherapy to the calf.
Adapted from Bierman, W., and Friedlander, M. 1940. The penetrative effect of cold. Arch Phys Ther, 21, 585.
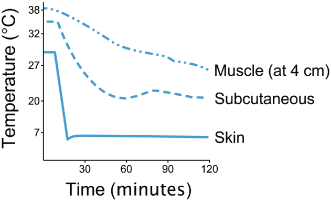
Deeper tissues require additional treatment time (Merrick et al., 2003). In addition, animals with more adipose tissue (a poor conductor of heat) will require increased treatment time to cool tissues that are deep to the layer of fat (Otte et al., 2002). Finally, colder agents (ice versus cold water) will lead to greater cooling (Herrera et al., 2010). Treatment time can range from 10 to 60 minutes (Otte et al., 2002), depending largely on the intensity of the cold modality. Drygas et al. found that the combination of cold with intermittent compression decreased signs of pain, swelling, and lameness, and increased stifle joint ROM in dogs during the first 24 hours after TPLO (Drygas et al., 2011).
For smaller treatment areas, ice massage is useful. It can be particularly effective prior to manual therapy techniques that may be uncomfortable. Ice massage is easily provided by freezing water in a paper cup, and tearing the paper away as the ice melts (Figure 7.4). Ice popsicles can also be made by placing a handle, such as a tongue depressor, into the water before freezing. The general recommendation for treatment duration is 5–10 minutes (Waylonis, 1967).
Indications
Cryotherapy is most often recommended during the acute stage of injury, but it can be effective throughout rehabilitation to reduce exercise-related inflammation and to treat arthritic joint pain.
Precautions and Contraindications
Caution should be used when applying cold over superficial peripheral nerves, areas of decreased sensation, or open wounds. Cryotherapy should not be used over areas of compromised circulation or in patients with thermoregulatory disorders or cold sensitivities. Patients should be monitored for signs of cold-induced injuries. Furthermore, areas previously affected by frostbite should not receive cryotherapy.
One final consideration: a cooled area can require an extended period of time to return to baseline temperature (Myrer et al., 1998). Cold can also increase joint stiffness (Uchio et al., 2003). These are important considerations when treating sporting or working dogs that will return to activity soon after treatment.
Superficial Heat
Superficial heat is used to reduce pain, promote relaxation, increase blood flow, and improve joint mobility and connective tissue flexibility. During the postacute and chronic stages of healing, heat may also reduce remaining inflammation through increased blood flow. Superficial heating modalities are used to increase tissue temperature up to 3 cm below the skin’s surface (Draper et al., 1998) although the greatest effects occur in the first 1–2 cm. Temperatures of the target tissue must be increased by 1–4°C to result in therapeutic effects (Draper et al., 1995).
Evidence Supporting the Use of Superficial Heat
Cutaneous blood flow is increased with superficial heat. Temperature changes are therefore most significant within 1 cm of the skin’s surface. Vasodilation occurs due to several mechanisms. When superficial tissue temperatures rise, chemical mediators are released that cause vasodilation. Cutaneous thermoreceptors release bradykinin, which relaxes smooth muscle walls. Finally, a reduction in sympathetic activation via spinal dorsal root ganglia also decreases smooth muscle contraction. Of note, superficial heat modalities do not significantly affect blood flow to skeletal muscle. Active exercise has a much greater effect. Superficial heating also increases the metabolic rate. When tissues are heated to within a safe range, oxygen uptake increases, accelerating healing (Halvorsen, 1990).
Increased tissue temperatures can increase nerve conduction velocity and decrease latency time for both sensory and motor nerves (Greathouse et al., 1989; Dioszeghy & Stalberg, 1992; Notermans et al., 1994) (Figure 7.5) and lower stimulus threshold for muscle spindle activity (Mense, 1978). These changes may promote an increased pain threshold (Lehmann et al., 1958) and help to relieve muscle-guarding spasm.
Figure 7.5 Change in action potential of sural sensory nerve.
Adapted from Greathouse et al.. 1989. Electrophysiologic responses of human sural nerve to temperature. Phys Ther, 69, 919. (With permission from the American Physical Therapy Association. This material is copyrighted, and any further reproduction or distribution requires written permission from the APTA.)

Finally, heat increases the viscoelastic properties of connective tissues. Superficial heating can therefore promote improved flexibility and joint range of motion (ROM) if the restricted ligament, tendon, or joint capsule is located superficially. In humans, use of superficial heat combined with stretching can be more effective than stretching alone (Robertson et al., 2005).
Considerations for Application of Superficial Heating
The most commonly used superficial heating agent in canine rehabilitation is a hot pack, including moist heat packs, gel packs warmed in hot water or a microwave, or clay packs (Figure 7.6). When using a hot pack, the patient should be positioned to encourage relaxation while permitting access to the treatment area (Figure 7.7). If needed, additional padding should be placed between the patient and the pack, and the skin should be checked frequently. Treatment duration is often 15–30 minutes and is typically performed before stretching or active exercise.
Indications
Superficial heat can be indicated after tissue healing has progressed to the subacute or chronic stages and the patient demonstrates decreased flexibility or joint ROM, pain, or chronic edema.
Precautions and Contraindications
Caution should be used over areas of decreased circulation, open wounds, or when treating sedated animals or those with decreased sensation. Contraindications include acute inflammation, active bleeding, fever, and applying heat over a malignancy.
Electrical Stimulation
The most commonly used forms of electrical stimulation (ES) in canine rehabilitation are neuromuscular electrical stimulation (NMES) and transcutaneous electrical nerve stimulation (TENS).
NMES
Neuromuscular electrical stimulation is often used to address muscular weakness. It can cause a muscle contraction by depolarizing the motor nerve with an external electrical current. The two common types of NMES units are portable, battery operated units and electric line-powered units. For most canine rehabilitation applications, portable units can provide sufficient output to achieve the desired therapeutic effect (Figure 7.8). Regardless of the type, most NMES units offer the same parameters of treatment although the terms used to describe each may vary.
Parameters of Treatment
Evidence Supporting the Use of NMES for Strengthening
Neuromuscular electrical stimulation is used to facilitate muscle strengthening and to retard disuse atrophy in patients who are unwilling or unable to actively contract a muscle. It is often noted that because NMES recruits muscle fibers in an order opposite to voluntary contraction (type II fast twitch before type I slow twitch), the muscle contraction will not be as strong as a maximal voluntary contraction in healthy individuals (Knaflitz et al., 1990). Many canine patients may not be able to maximally contract a muscle after injury or surgery. In these cases, an electrically induced muscle contraction may produce greater torque—and therefore greater strength gains—than if NMES were not used (Fitzgerald et al., 2003). Clinically, there is evidence that NMES use following orthopedic surgery results in decreased muscle mass loss, increased muscle strength, and improved functional muscle use (Snyder-Mackler et al., 1991).
TENS
When used for pain relief, ES is referred to as TENS. Most commonly, TENS is used to provide sensory-level stimulation, known as conventional TENS, to stimulate sensory nerves rather than motor nerves (Figure 7.9). This is believed to reduce pain perception through the gate theory of pain inhibition. This may then enable patients to participate more fully in their rehabilitation program. However, pain relief is provided only during stimulation. Overall, research results have been mixed regarding the ability of TENS to effectively reduce pain.
Considerations for the Clinical Application of ES
Safety should always be considered when working with animals. Muzzling may be recommended, at least for the first ES treatment to allow assessment of the patient’s tolerance for the modality.
The general technique for providing NMES is to place the electrodes over the muscle(s) to be stimulated (Figure 7.8). A coupling medium is needed to transmit the current from the electrode to the tissues; some electrodes are coated with a conductive polymer, while carbon silicon-rubber electrodes are used with an aqueous gel. Especially in dogs with thicker coats, the hair may need to be clipped to improve transmission. The size of the electrodes should fit the target muscle. Small electrodes are available to accommodate small treatment areas (Figure 7.10), but can cause discomfort with higher amplitudes due to the greater current density. The electrodes used should be as large as possible to maximize comfort while avoiding overflow of current to other muscles. The electrodes should also not contact each other, nor should their coupling medium, as this will result in the current flowing directly from one electrode to the other instead of into the patient’s tissues.
Figure 7.10 Examples of various ES electrodes.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







