Fig. 1.1
Title I of the FSMA concerns the improvement of the capability of preventing food safety problems by means of different actions: the inspection of records concerning ‘suspect’ foods; the registration of the U.S. and foreign food plants; mandatory preventive controls for food plants on the basis of Hazard Analysis and Risk-based Preventive Controls (HARCP); the creation of performance standards (with reference to the most important foodborne contaminants); the definition of ‘Standards for Produce Safety’ (safe production and harvesting of fruits and vegetables); the enhancement of actions against intentional adulteration; the creation and implementation of detailed sanitary transportation practices
(a)
Inspection of records. Substantially, the Department of Health and Human Service (HHS) through FDA can inspect documents concerning ‘suspect’ foods. The documentation may not be directly related to food products (analytical reports): on the contrary, the attention is focussed on manufacturing, processing, packaging and subsequent pest processing steps (distribution, importation, etc.)
(b)
Registration of food plants. The U.S. approved foods and feeds have to be produced and commercialised by registered food facilities (the registration has to be renewed every 2 years). Should foods be imported, the role of the U.S. agent for the foreign facility would be considered as contact person. The registration can be suspended unless an adequate corrective action plan has to be taken and immediately implemented if a certain food linked with a registered facility may hypothetically cause serious adverse health consequences or death to humans or animals
(c)
Hazard Analysis and Risk-based Preventive Controls (HARCP). This section concerns mandatory preventive controls for food plants and the new HARCP strategy, with the important exclusion of ‘small’ and ‘very small business’ activities. Certain ‘on-farm’ and operations defined as farms are also not subject to the preventive control rules
(d)
Performance standards. This section focuses on gaining a greater understanding of foodborne contaminants and correlated guidelines. These documents will be issued every 2 years
(e)
Standards for Produce Safety. The HHS is directly involved in the publication of mandatory minimum standards for the safe production and harvesting of fruits and vegetables with the important exclusion of ‘small’ and ‘very small business’ activities
(f)
Protection against intentional adulteration. This section has been developed as a tool to provide effective actions against food frauds
(g)
Sanitary Transport (detailed sanitary transportation practices).
1.3.3 The FMSA: Responsiveness to Food Safety Crises
The second title of the FSMA is focused on the enhancement of the responsiveness when food safety problems are detected and an immediate action is required (Johnson 2014b). The following sub-topics discussed below apply directly to the aim of improving responsiveness to food safety crises (U.S. Congress 2011):
(a)
Inspection Resources. The FDA identified ‘high-risk’ plants with the concomitant need for augmenting the number of inspections on domestic and foreign facilities. In addition, ports of entry are crucial from the point of view of maintenance of the food safety chain, in addition to audits
(b)
Tracking and tracing food records. With reference to high-risk foods and product tracing/tracking systems to improve the overall recall efficiency
(c)
Surveillance. The assessment of the U.S. state and local food safety and defense capabilities are considered as one of the pillars of the FSMA
(d)
Mandatory Recall Authority. The FDA has received new powers referring to its ability to order food recalls ‘under certain circumstances’.
(e)
Administrative Detention of Food. Under this criterium, FDA can commission detention of edible products that it identifies as being adulterated or misbranded
(f)
Decontamination and Disposal Standards and Plans. This section highlights the role of EPA in spearheading the support and technical assistance in clearance and recovery activities following decontamination and disposal of specific threat agents and foreign animal diseases.
1.3.4 The FSMA and Imported Foods: Consequences for Foreign Suppliers
The third title of the FSMA deals directly with the safety of imported foods, including fresh fruits and vegetables (Johnson 2014b; Kelley Drye 2011; U.S. Congress 2011). Basically, the new approach requires that foreign food business operators (FBO) comply with the FDCA (amended by the FSMA) before the commercialisation of food products and the consequent arrival of edible commodities in the U.S. (Kelley Drye 2011). For this reason, each importer—the foreign FBO or the U.S. agent—has to consider certain measures with the aim of mitigating food safety risks and gather evidence of the required compliance. The following modules of FSMA are significant from the point of view of bolstering the objectives of Title III (Akingbade et al. 2014; Johnson 2014b; Keenan et al. 2015; Kelley Drye 2011; U.S. Congress 2011):
Foreign supplier verification program and certification
Third-party certification bodies and correlated accreditation
Establishment of foreign FDA offices
Agreements with foreign governments and other actions.
With relation to basic aims of this book, some of the above mentioned points need to be discussed carefully in the following sections because of the mandatory controls that have to be in place for fresh fruits and vegetables. Other arguments without mandatory requirements are not discussed here.
As an example, the expedited entry of certain foods is correlated with the creation and the implementation of a voluntary program for qualifying those specific U.S. food importers. In addition, the role of third-party certifications will be equally important, especially considering the fact that all importers may not be able to demonstrate maintaining a high level of controls over the safety and the security of their supply chains.
1.3.4.1 Foreign Supplier Verification Program and Certification
Basically, foreign food business operators involved in the exportation of their own products to the U.S. have to assure the legal compliance of every U.S. imported product in adherence to FDCA requirements. Dedicated guidance documents are provided by the FDA with the aim of helping importers. In fact, the creation and the implementation of the FSMA rule for foreign supplier verification programs (FSVP) for importers of food for humans and animals are dedicated to this objective. However, seafood, low-acid canned foods, fruit juices and other edible products currently already subject to the ‘Hazard Analysis and Critical Control Points’ (HACCP) approach are exempted from the FSVP requirement (U.S. Congress 2011). Similarly, foods imported for research purposes in small amounts may be imported without being subject to FSVP (Kelley Drye 2011). All other foods have to be commercialised in the U.S. with an implemented supplier verification program in place. However, the deadline for these provisions is 2 years after the enactment of the FSMA. At present, general rules should be in force between the last part of 2016 and beginning of 20171 (Keenan et al. 2015).
The publication of the final FSMA rule for FSVP is set for 3 October 2015 (Keenan et al. 2015). In the meantime, the U.S. food importers should (Fortin 2015; U.S. Congress 2011):
Consider the review of their food imports and corresponding suppliers with relation to compliance standards
Define and implement a careful analysis of hazards
Carry out audits and/or different surveillance actions on suppliers
Define and perform corrective activities to ensure that their suppliers are ‘approved’
Create and/or implement and update records of performed actions as per to FDCA requirements.
1.3.4.2 The Role of Third-Party Certification Bodies
The FDA may be assisted by third-party certification bodies when particular food products need to be defined as ‘compliant’ with the requirements of the U.S. food safety legislation (Kelley Drye 2011). Moreover, these actions of the certification bodies can be specific for single shipments, or focused towards getting the ‘source’ (food facilities). Parallely, certifications issued by certain institutions may be accepted on condition that the U.S. established standards are observed. Should the FDA consider this option (the third-party certifications), the action of non-FDA entities would be restricted to high-risk foods only. In addition, certification and related documents must be scientifically reliable and the analysis of known food safety risks has to be considered (Keenan et al. 2015; Kelley Drye 2011). FDA has created and implemented a particular user fee-based program for the accreditation of domestic and foreign third-party auditors and certification bodies.
1.3.4.3 Establishment of Foreign Inspectorates and Other Actions
The FDA plans to increase its own activity in foreign countries by means of the creation of FDA offices. This measure has two distinct aims:
(1)
The enhancement of communication with the non-U.S. institutions and agencies
(2)
The augmentation of a powerful transnational cooperation, in spite of known differences (language, cultural behaviour, regulations, etc.).
At the same time, the FDA can establish synergic agreements with foreign institutions with the aim of raising the number of ‘actual’ audits and inspections, with greater emphasis on high-risk foods (Kelley Drye 2011). In fact, the sheer number of foreign inspections is really challenging for FDA staff: the predicted number of these activities is anticipated to be 19,200 in 2016 (Fortin 2015).
In addition to the creation of a detailed plan in strong cooperation with foreign authorities, partnerships and working relationships with food industries and consumers associations will be equally valuable to ensure food safety of edible goods from foreign countries (Kelley Drye 2011).
1.4 Possible Consequences of the U.S. Imported Fresh Fruits and Vegetables on the Market
On the basis of a previous discussion of FSMA modules, it can be concluded that the U.S. as well as foreign food producers must take steps towards the upcoming requirements in a coherent manner. Briefly, the following points may concern fresh fruits and vegetables industries, with a specific focus on imported foods as a consequence of the strengthening of FSMA rules (Fig. 1.2):
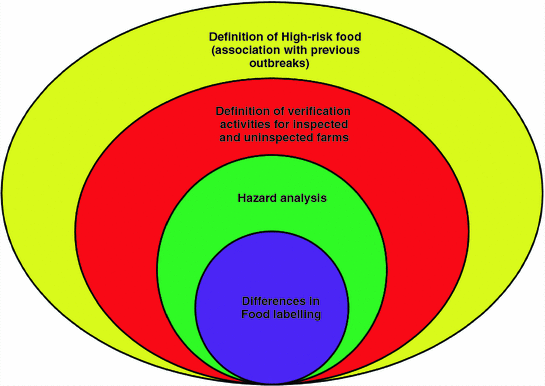

Fig. 1.2




U.S. as well as foreign food producers must take steps towards the upcoming requirements in a coherent manner. The following points may concern fresh fruits and vegetables industries, with a specific focus on imported foods as a consequence of the strengthening of FSMA rules: the definition of ‘high risk’ fruits and vegetables (this definition has to be associated on the basis of previous outbreaks); the definition of verification activities for inspected and uninspected farms; the mandatory application of hazards analysis; the different applications of food labelling requirements
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


