CHAPTER 1 Radiology Equipment and Positioning Techniques
ANATOMIC REFERENCES
Anatomic drawings demonstrating the spatial relationship of the internal organs are provided in Chapter 2. They should be used as general reference material. Individual organs are not always clearly visualized on all radiographs. There are species variations in the size, shape, and location of internal organs. The radiographic appearance of the viscera is also affected by the birds’ reproductive status and digestive tract contents. In the case of this text, the reproductive organs were labeled as “gonads” (versus ovary or testes) if no specific anatomic structure (i.e., the syrinx in male duck) could be identified on the radiograph to indicate the bird’s sex.
EQUIPMENT FOR RADIOGRAPHIC STUDIES
RADIOGRAPHIC FILM-INTENSIFYING SCREENS
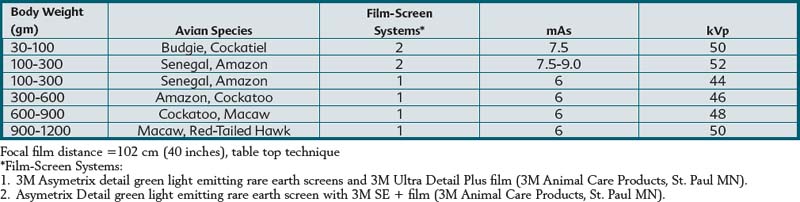
Selection of radiographic film and intensifying screens is based on the speed of the system (i.e., mAs required to produce a high-quality diagnostic image). Film-screen speed and detail are inversely related. Faster systems are usually capable of producing less detail than slower systems. Asymetrix Detail Intensifying Screens (3M Animal Care Products, 3M Center, St. Paul, Minn.) and Ultra Detail Plus or SE + radiographic film (3M Animal Care Products, 3M Center, St. Paul, Minn.) were used to produce the radiographic images in this book. This film-screen combination produced a radiographic system speed of 100 to 350. Table 1-1 summarizes radiographic exposure factors used for creating this text’s radiographic images using a tabletop technique. These settings are intended to be guidelines and may require modification depending on the x-ray generator, film-screen combination, radiographic film processing, and patient size. Other film-screen combinations of similar speed and resolution can also be used if they are of sufficient detail and speed.
THE RADIOGRAPHIC EXAMINATION
ANESTHESIA
Radiographic studies for which the birds are anesthetized with inhalation gas anesthetics are generally completed in less time and are of higher quality than studies in which the birds are not anesthetized. Anesthetized birds are easily positioned with less physical restraint, and the potential for iatrogenic fractures is minimized. It is also possible to inflate the air sacs with positive pressure ventilation in the intubated bird. Motion artifacts are also reduced with anesthesia. All birds in this text were healthy, and the majority of the studies were performed using inhalation anesthesia or chemical sedation. Birds seen in clinical practice may be severely debilitated, and general anesthesia may be contraindicated; however, it is recommended whenever the anesthetic procedure is deemed safe.
POSITIONING DEVICES
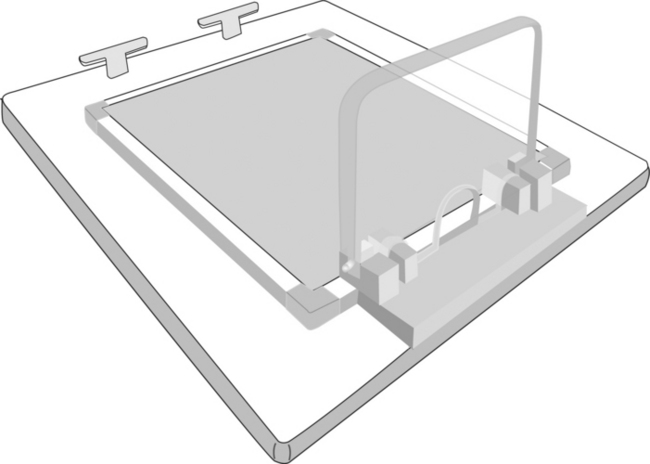
For production of the images for this text, smaller birds (i.e., less than 100 grams body weight) were positioned directly on the radiographic cassette and secured with masking tape, but an acrylic positioning device (Bird Board, 8205 Alba Ct., Citrus Heights, Calif.) was used to facilitate positioning of larger birds. Many avian positioning devices are commercially available. If positioning devices are interposed between the bird and the film or digital sensor, a small increase in kVp (2–4 kVp) may be required to compensate for x-ray beam filtration caused by the device. The need for exposure compensation is especially necessary with low kVp techniques (40–50 kVp) and thicker positioning devices. Positioning devices placed on the x-ray cassette or used with a digital system increase the object film or sensor distance. The increased object film or sensor distance may decrease image detail, but the magnitude of loss is usually minimal. A modified version of the Bird Board is available that allows for direct contact of the bird and the radiographic cassette (Figure 1-1).
PATIENT POSITIONING
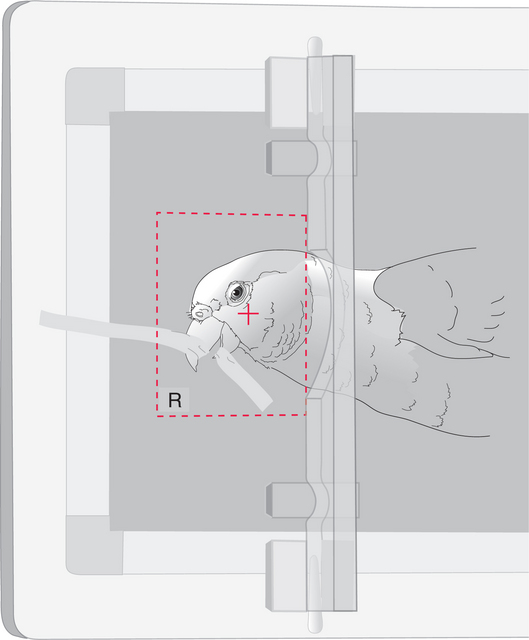
POSITIONING TECHNIQUES FOR LATEROLATERAL AND VENTRODORSAL RADIOGRAPHIC STUDIES OF THE AVIAN HEAD
Radiographic studies of the head include laterolateral and ventrodorsal radiographic projections and, when necessary, oblique views. Small wedges of radiolucent foam may be of assistance for precise positioning of the head. For laterolateral and oblique projections, the patient is placed in a lateral recumbent position. Oblique radiographic projections require rotation of 15 to 30 degrees or less off the straight lateral projection. Oblique projections are described by the point of entrance of the x-ray beam to the point of exit. For ventrodorsal projections, the patient is positioned in dorsal recumbency and tape is applied to the ventral aspect of the rhinotheca so that the maxilla is closer to the cassette. This positioning of the head changes the orientation from a rostrocaudal to a ventrodorsal projection (Figures 1-2 to 1-3).