CHAPTER 19 Rabies
Rabies virus (RABV) is an enveloped ribonucleic acid (RNA) rhabdovirus that induces lethal polioencephalomyelitis and ganglionitis in infected animals.1,2 The disease is universally endemic in mammals and other warm-blooded vertebrates, except in Australia, where other types of zoonotic lyssaviruses transmitted by flying foxes (bats) are present. The disease has been excluded or eradicated from some countries, or parts of them, especially islands (e.g., Great Britain, New Zealand, Iceland).
ETIOLOGY
Viruses in the Rhabdoviridae family known to infect mammals belong to either the genus Vesiculovirus (vesicular stomatitis virus serotype New Jersey and serotype Indiana, Chandipura virus, and Piry virus) or the genus Lyssavirus (rabies virus and rabieslike viruses).1,2 Lyssaviruses include six distinct genotypes that can be classified according to their degree of amino acid homology. Genotypes 2 (Lagos bat virus) and 3 (Mokola virus) are the most phylogenetically distant from the vaccinal and classic rabies viruses of genotype 1. Genotypes 4 (Duvenhage virus) and 5 (European bat lyssavirus 1 [EBL1]) are closely related to each other, with the separate genotype 6 represented by EBL2.
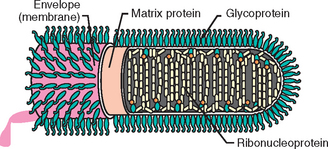
RABV virions are enveloped, bullet-shaped, 45 to 100 nm in diameter, and 100 to 430 nm long (Fig. 19-1). Surface projections of the envelope are distinct spikes, dispersed evenly over the whole surface (except for the quasiplanar end of bullet-shaped viruses). The uncoiled nucleocapsid is filamentous, with regular surface structure, and cross-banded. Virions contain 1% to 2% nucleic acid composed of one molecule of linear, usually negative-sense, single-stranded RNA. Nucleotide sequences of the 3′ terminus are inverted and complementary to similar regions on the 5′ end and are the same for each gene segment in species of the same genus. Virions contain 65% to 75% protein, most of which are structural. RABVs are recognized and classified through panels of monoclonal antibodies against nucleocapsid proteins. The pattern of antiglycoprotein reactivity of the isolates allows identification of the viral subtype.
EPIDEMIOLOGY
RABV is transmitted to warm-blooded animals by bites from infected vectors such as foxes, raccoons, skunks, bats, and vampire bats. In 2003, wild animals accounted for more than 91% of all cases of rabies reported to the Centers for Disease Control and Prevention (CDC).3 Rabies control programs, including vaccination programs, have significantly decreased, if not eliminated, rabies in humans caused by canine variants. However, ever-increasing numbers of human cases are attributable to bat variants, a group difficult to target for rabies control by traditional methods.
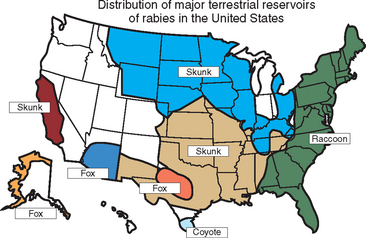
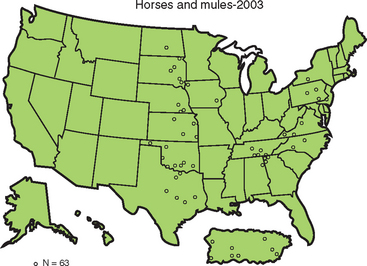
In the United States, rabies infection of terrestrial mammals occurs in geographically defined regions, and transmission is usually within species, with occasional spillover to other species that rarely maintain intraspecific transmission (Fig. 19-2). Sixty-three cases of rabies were reported in horses and mules (including donkeys) in 2003, an 8.6% increase over the 58 cases reported in 2002 (Fig. 19-3). The majority of rabies in horses occurs in animals with no history of vaccination, although it may be recognized in animals not vaccinated within a year of diagnosis.4 The increase in equine rabies in 2003 was consistent with the increase observed in raccoons, 8.9%, while the number of rabid skunks decreased by about 13%.
PATHOGENESIS
The primary means of transmission of RABV is through the bite of an infected animal that inoculates saliva containing virus into tissues of a receptive animal. The virus likely replicates in muscle tissue at the site of inoculation. Rabies virus is believed to remain near the site of initial inoculation for most of the incubation period. In 1889, DiVestea and Zagari5 showed that mortality was greatly diminished by severing the sciatic nerves of experimental animals before virus was inoculated in the footpad. This reduction in mortality is seen even when the nerves are severed several days after inoculation of virus.6 Delayed progression of infection within muscle cells is thought to be responsible for the long incubation period of clinical rabies.7
Eventually, RABV binds to nicotinic acetylcholine receptors at the neuromuscular junction, the major site of entry into neurons.8,9 The neural cell adhesion molecule, the low-affinity p75 neurotrophic receptor, and perhaps the N-methyl-d-aspartate NR1 receptor may also function as RABV receptors.10–13 The virus then travels in an ascending fashion through the spinal cord to the brain or from the cranial nerves directly to the brain stem. Once transit in peripheral nerves begins, it progresses rapidly through transsynaptic neuronal spread. Virus can be observed in peripheral nerve myelin late in infection.14 Clinical signs appear to result primarily from neuronal dysfunction secondary to drastically diminished synthesis of proteins required for maintenance of normal neuronal function.15 After it reaches the brain, RABV is passed centrifugally to tissues and organs. It reaches the salivary glands and nasal secretions after passage down appropriate cranial nerves.16
CLINICAL FINDINGS
Several authors have described clinical signs of rabies in horses in detail.4,17–19 These signs range from poor racing performance to bizarre behavior and may include spinal cord, cerebral, and cranial nerve signs; apparent lameness; gastrointestinal signs; and genitourinary signs (Fig. 19-4). Clinical signs are progressive from onset until death, which usually occurs by day 10. Average survival from the onset of clinical signs is approximately 5 days. Clinical chemistry and hematology results are nonspecific and nondiagnostic. Cerebrospinal fluid (CSF) analysis reveals increased protein and lymphocytes but is not diagnostic.