CHAPTER 14 RABBITS
Domestic rabbits, Oryctolagus cuniculus, belong to the order Lagomorpha, and their ancestors are from Western Europe and northwestern Africa.1,2 Unlike rodents, lagomorphs have a second set of maxillary incisors directly caudal to the first set. Pet rabbits have unique, lively, and affectionate personalities that make them ideal pets for mature children and adults. Handling pet rabbits when they are young will likely make them more comfortable with humans later in life. In contrast, wild rabbits, no matter their age, do not become comfortable with human interaction. Rabbit owners commonly seek medical attention for their rabbits and husbandry guidance from veterinarians.
COMMON BREEDSKEPT IN CAPTIVITY
There are as many as 50 different breeds of domestic rabbits.3 However, many of the rabbits presented to private practice are mix breeds. Breed classification can be divided by size or fur type. The size classification is more helpful to the practicing veterinarian and can be divided into small breeds, less than 2 kg (e.g., Netherland dwarfs, lion head, mini-lop, and Dutch breeds); medium breeds, 2 to 5 kg (e.g., Rex, English, Angora, and Belgium hares), and large to giant breeds, more than 5 kg (e.g., New Zealand whites, English lops, British Giants, and Flemish Giants). The American Rabbit Breeders Association publishes information on the different breeds and various standards, and is an excellent resource for veterinarians (http://www.arba.net/).
Certain breeds are predisposed to health problems. For example, dwarf breeds, such as the Netherland rabbits, have short maxillae that make them more prone to nasolacrimal duct blockage and incisor malocclusion. In addition, dwarf breeds appear more susceptible to Encephalocytozoon cuniculi infections.2 Dutch, Havana, tan, and French silver breeds are more likely to develop uterine neoplasia.4 Because Rex rabbits have thin fur on the plantar surface of their feet, they are more susceptible to developing hock sores. Large and giant breeds are prone to developing arthritis and cardiomyopathies. British Giant and French lops are more likely to develop skin fold pyoderma under their dewlaps and in the perineum area, in addition to developing entropion.2
BIOLOGY AND PHYSIOLOGY
BOX 14-1 Physiologic Parameters for Captive Rabbits
| Life span | 6-13 yr |
| Gestation | 30-32 days |
| Ages of weaning | 4-6 wk |
| Age of puberty | Small breeds 4-5 mo |
| Large breeds 5-8 mo | |
| Age of recommended neutering | Castration >3 mo, spay >5 mo |
| Temperature (rectal) | 37.8°-39.4° F (100°–103° F) |
| Heart rate | 130-325 beats/min |
| Respiratory rate | 32-60 |
Sense Organs
Rabbits’ ears represent a large amount of the animals’ surface area. Because rabbits are low in the food web, these large ears serve an important function in assisting the animal with identifying potential predators. In addition, rabbits’ ears are highly vascularized and serve an important role in thermoregulation via vasoconstriction and vasodilatation.5 Rabbit ears are very sensitive and delicate and should never be used for restraint.
Rabbits have a wide field of vision, which is ideal for a prey species. With the lateral positioning of their eyes and their large corneas, rabbits have an overlapping field of vision of 190 degrees.3 Although rabbits are visually acute within this region, they cannot visualize items below the horizon, including the area below their nose. Instead, rabbits use their highly sensitive vibrissae and lips as tactile structures to distinguish food items. Rabbits possess good night vision and some color vision. Rabbits have functional third eyelids that partially close with sleep or while under anesthesia.5 A harderian gland is found at the base of the third eyelid. If this gland prolapses, then it may present as a mass under the third eyelid. Surgical intervention is recommended to replace the gland. Tear drainage from the eyes occurs via the nasolacrimal gland. There is a single lacrimal punctum located in the craniomedial aspect of the lower eyelid. The punctum empties into a cuniculus, leading to a lacrimal sac and continuing to the base of the maxillary incisor. At this point, the duct sharply changes direction, runs along the incisor root, and emerges in the nasal cavity in the ventromedial aspect of the alar fold.2 Blockage of this duct is common in captive rabbits. Affected animals may have unilateral or bilateral epiphora. Rabbits have a large retrobulbar orbital venous plexus. When an enucleation is performed, this venous plexus must be ligated to minimize hemorrhage.
Musculoskeletal System
Rabbits have a light skeleton surrounded by a very well-developed muscular system, which makes the vertebrae and long bones susceptible to fractures. To minimize the likelihood of injury, the hindlegs should always be supported when transporting the rabbit. The number of vertebrae varies between breeds, from 12 to 13 thoracic and 6 to 7 lumbar vertebrae.6 The epaxial and large muscles of the hindlimbs can be used for intramuscular injections.
Reproductive System
Female rabbits are classified as does, males as bucks, and neonates as kits. The onset of puberty occurs at approximately 4½ months of age; however, reproductive activity is generally dependent upon animal size, not age. In the dwarf breeds, sexual maturity occurs earlier than in larger breeds. The reproductive life of a rabbit is approximately 4 years.1 Rabbits are induced ovulators. Their receptivity periods last approximately 14 to 16 days, followed by 1 to 2 days of nonreceptivity.2 When females are ready to ovulate, they become restless, rub their chins, and assume a lordosis-like posture. Gestation lasts 31 to 32 days, and litters average 4 to 10 kits. As gestation or pseudopregnancy reaches the last 3 to 4 days, does initiate nesting behavior and line their nests with hair from the hip and dewlap.3 During this period, does consume less food, which can cause an increased risk for pregnancy toxemia. Parturition usually occurs in the early morning and lasts less than 30 minutes. Kits generally only suckle 1 or 2 times a day and are not usually seen with the doe, especially in the wild. Weaning occurs at approximately 4-6 weeks of age.
Urinary System
The kidneys of rabbits are located in the retroperitoneal cavity, and their position in the body cavity is similar to that in cats. The kidneys are generally covered in fat and can be palpated on the physical examination. The urinary bladder is located in the ventral body cavity, and it is ventral to the colon. Rabbits, like rodents, have a unique system for calcium metabolism. Serum calcium is reflective of dietary calcium, and the urinary system is the primary site of calcium and magnesium excretion. Rabbits that consume a high calcium diet will often have thick and creamy urine due to the formation of calcium carbonate precipitate. On average, rabbits produce 30 to 35 ml of urine daily. Rabbit urine may be straw-colored, yellow, or red. It is not uncommon for rabbit urine to have a turbid appearance. When a rabbit presents with “red” urine, it is important to differentiate whether the urine has blood or porphyrins. Porphyrins can be produced as a result of the ingestion of various vegetables, such as beet root, cabbage, broccoli, and dandelions, or as a result of stress.2
Gastrointestinal System
Rabbits have open-rooted teeth that grow continuously throughout their lives at a rate of approximately 10 to 12 cm a year.1 The dental formula of the rabbit is 2 × (2/1 I, 0/0 C, 3/2 P, 3/3 M). Directly caudal to the maxillary incisors is a second pair of small incisors called peg teeth, whose function is to protect the palate from injury against the sharp surfaces of the lower incisors. The buccal surface of the upper incisors’ enamel is thicker and wears more slowly than the rest of the tooth, forming a chisel-like appearance. Caudal to the incisors is a space called the diasterna.7 The cheek teeth are located caudal to the diasterna and are responsible for macerating and grinding food.
Rabbits have simple, glandular stomachs. Due to the positioning and well-developed nature of the cardiac sphincter, rabbits are unable to vomit.8 Adult rabbits have a gastric pH of 1.5 to 2.2, whereas suckling rabbits have a higher pH of 5.0 to 6.5. The higher gastric pH in neonates is necessary for bacterial colonization of the intestinal tract. This elevated pH also makes suckling rabbits more susceptible to gastrointestinal disease. Dietary absences, especially during weaning, should be gradual, and foods high in sugars and starch should be avoided. Rabbits have a large cecum that acts as an anaerobic fermentation vat. The cecum has a complex and delicate population of microbes. The most common organisms found in the rabbit cecum are the Gram-positive Bacillus spp. In the cecum, fiber is broken down and separated. Rabbits are highly susceptible to antibiotic-induced enterotoxemia, especially when there is Clostridium spiriforme contamination in the environment. The rabbit’s gastrointestinal tract is designed to eliminate fiber from the gut rapidly, in the form of hard feces, and digest the nonfiber portion of the diet, creating caecotrophs, which are reingested at a later time. Cecotrophs are covered with a thin mucus, which protects the volatile fatty acids, vitamins, and amino acids from the low pH of the stomach. Cecotrophs differ from feces in that they are soft, moist, usually stuck together, and are eaten directly from the anus.8 The gastrointestinal transit time in rabbits is approximately 20 hours. Rabbits offered a diet deficient in fiber usually have increased gastrointestinal transit times.
Respiratory System
Rabbits are obligate nasal breathers. A rabbit that presents for open-mouth breathing generally has a guarded prognosis. The respiratory rate of the rabbit is approximately 30 to 60 breaths per minute. In a healthy animal, the nose will move up and down approximately 20 to 120 times a minute. The thoracic cavity of the rabbit is small in comparison to that of nonlagomorph or rodent mammals. Rabbit lungs have four right and two left lung lobes.3 Rabbits have an extremely narrow and long oral cavity. Because of these features, it can be difficult to visualize the glottis for intubation. Endoscopic examination provides the best visualization of the glottis for intubation. Inflammation of the upper respiratory tract increases the risk of anesthetic mortality in nonintubated animals.4
Cardiovascular System
Rabbits have a relatively small heart, and the average heart rate of the rabbit is 130 to 325 beats per minute.9 Systolic blood pressure is approximately 90 to 120 mmHg. The right atrioventricular valve has only two cusps, and the aorta rhythmically contracts.4 Rabbit veins are thin and susceptible to hematomas, so care must be taken with venipuncture.
HUSBANDRY
Environmental Considerations
ENCLOSURE SIZE
A rabbit enclosure should be large enough to provide a sleeping space, eating space, and latrine. Animals housed for long periods of time should also have ample room to exercise. The enclosure should be tall enough to allow the rabbit to sit up and not have its ears touch the top of the cage. In general, 3 square feet (sq ft) should be a minimum cage size for rabbits 2 to 4 kg, 4 sq ft for rabbits 4 to 4.5 kg, and larger rabbits require at least 5 sq ft.5 Rabbits housed in hutches and cages should be allowed a minimum of 4 hours of exercise daily.2 Indoor rabbits should be caged when they cannot be supervised, to decrease the likelihood of inappropriate chewing of carpet, electrical wire, or furniture. Cages should be cleaned daily to remove feces and urine. Gentle soap and hot water or a dilute bleach solution (1 : 32) should be used for cleaning. If bleach is used, then the cage should be cleaned in a well-ventilated area. Organic material should be removed before applying the bleach, to minimize the degradation of the bleach. The bleach or soap should have a minimum of 3 to 5 minutes of contact time on the cage to increase the killing potential of these products. It is important to clean, rinse, and dry off all detergents.
TEMPERATURE/HUMIDITY
Because rabbits are prone to heatstroke, they should be housed in temperatures ranging from 60° F to 75° F.2 If rabbits are housed outdoors, they must have access to shade and clean water, and the shelter should protect them from the elements as well as from predators. Rabbits are prone to developing fur and skin problems when housed in environments with high humidity; therefore, these animals should be provided environments with low to moderate humidity (30%-60%).
LIGHTING
Rabbits show a diurnal rhythm for eating, activity, and even hematologic variation. Most feeding takes place from after noon to evening. During foraging, rabbits commonly produce fecal pellets; in times of decreased intake, rabbits will actually ingest cecotrophs. In addition, hematologic parameters, such as the total white blood cell count, are lowest in the late afternoons and evenings. Although the eosinophils peak in early afternoon, the heterophils are highest in the late afternoon and early evening. Other changes can be seen with diurnal patterns, including changes in blood urea nitrogen, body temperature, and bile acid production.2 Because much of the rabbits’ behavior and physiology is regulated by diurnal patterns, it is important to provide a consistent light cycle. Although there are no specific lighting recommendations for rabbits, nonreproductive rabbits should be provided a 12-hour photoperiod, and breeding females should be provided 14 to 16 hours of light.10
SUBSTRATE
The ideal substrate for rabbits is grass hay; however, for indoor, caged rabbits, a foam rubber pad or a towel covered with newspaper and a thick layer of timothy hay can also be used.2 Avoid wood shavings, which contain oils that can lead to hepatotoxicity.2 Rabbits can easily be trained to use a litter box filled with straw, hay, or newspaper litter. It is important to keep any substrate clean and dry. If a rabbit is housed on a wire mesh floor, it is important that the mesh openings are no larger than 1 × 2.5 inches to prevent the feet from getting trapped and injured. In addition, it is also important to offer a solid, nonslip surface to provide rest off the mesh and prevent pododermatitis.4
Nutrition
An average adult house rabbit should be offered ad lib grass hay, such as timothy, prairie, or oat brome; approximately 1 cup of leafy green vegetables; and, at most, ¼ cup of high-fiber (18%-22% to prevent obesity), low-protein (<18%) pellets per 2.2 kg (5 lbs) of body weight daily.4,8 Examples of dark leafy greens include romaine lettuce, dandelion greens, Swiss chard, parsley, endive, kale, mustard greens, and carrot, beet, and turnip tops. Diets low in fiber, such as commercial, pelleted rations, may be associated with hypomotility, changes in the gastrointestinal pH and microflora, wool block from increased hair consumption, and cheek tooth overgrowth.7 Alfalfa hay should be avoided in adult animals, because of its high protein and calcium content, and instead, reserved for animals less than 6 months of age and for pregnant and lactating does with increased nutritional demands. When offering alfalfa hay to lactating does, slowly increase the amount over the first 5 days, as this will reduce the likelihood of milk overproduction and decrease the chance for the development of mastitis.4 Any diet change should be gradual.
Chlorinated water should always be in fresh and ample supply. Rabbits are very sensitive to water deprivation and therefore should experience no restriction. Rabbits generally drink 50 to 100 ml/kg/day.2 Water should be offered in a nonleaking sipper bottle or in a heavy crock that cannot be tipped over. Rabbits that drink from crocks may develop “blue fur,” which is a moist dermatitis of the dewlap that is associated with Pseudomonas infections.
PREVENTIVE MEDICINE
Rabbits’ enclosures should be cleaned on a daily basis. Organic material should be removed before disinfectant is applied. Fresh substrate should be offered either daily or as it is soiled. The cage may be disinfected with mild soap and water or a dilute bleach solution (1 : 32). The disinfectant should have a minimum of 3 to 5 minutes of contact time to increase the effectiveness of the compound. The solutions should be thoroughly rinsed away, and the surface of the cage dried completely. Roccal D or chlorhexidine (Fort Dodge Inc., Fort Dodge, IA) can also be used to disinfect rabbit enclosures.
RESTRAINT
Manual Restraint
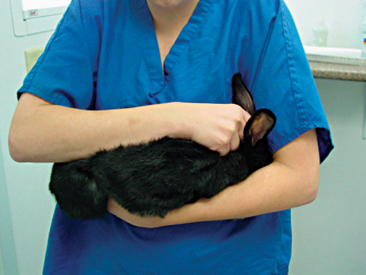
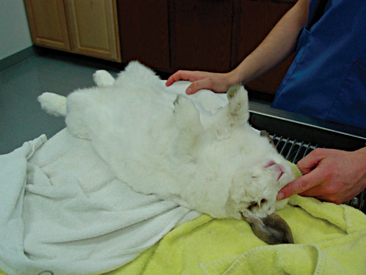
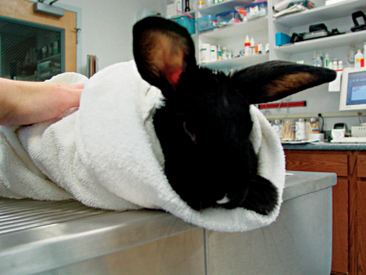
Rabbit physical restraint needs to be carefully performed to avoid injury to the animal. Because rabbits have a well-developed muscular system and thin cortical bone, they are subject to vertebral and long bone fractures if restrained incorrectly. Because most skeletal injuries associated with incorrect restraint occur in the lumbar vertebrae, it is important to firmly restrain the hindlegs. Rabbits should be handled in a manner similar to cats; place one hand on under the forelimbs, and use the other hand to hold the rear legs against the body. Always place the rabbit onto a nonslip surface to ensure that it has good footing. To restrain the animal, lightly scruff the animal and support its dorsum with the same arm. The opposite arm is used to support the body and rear legs (Figure 14-1). It can be helpful to tuck the head of the rabbit into the crook of one’s elbow to decrease the rabbit’s vision, which can decrease the stress on the animal. Placing a rabbit in dorsal recumbency evokes an immobility response, which can be helpful during simple procedures. However, this response is performed by prey species under stressful or threatening conditions and may not be welcomed by all rabbits (Figure 14-2). The immobility response is characterized by a lack of spontaneous movement and a failure to respond to external stimuli. This restraint technique should only be used in those cases where chemical sedation is not desired.2 A towel or blanket is usually necessary during the physical examination to cover the stainless steel exam table and to minimize slipping or kicking, which can lead to injury. In addition, a towel may be used to wrap the rabbit into a “bunny burrito” (Figure 14-3). The bunny burrito can be used to hold a rabbit for transport or to facilitate the examination of the head. Cat bags can also be used to restrain rabbits. In addition to using these techniques for examination, they can also be used to administer medication, collect blood samples, or perform nail trims.
PERFORMING A PHYSICAL EXAMINATION
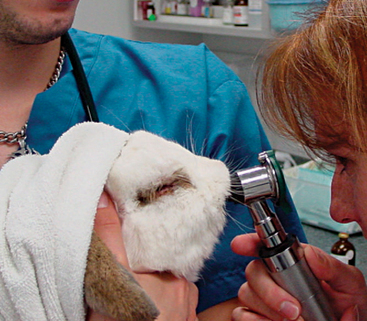
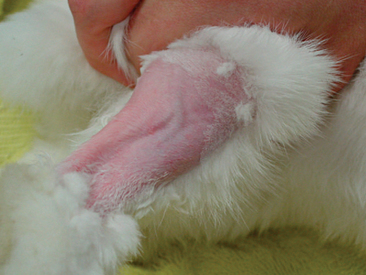
A thorough oral examination is an important component to any rabbit examination. Rabbits commonly present to the veterinarian with malocclusions of both incisors and cheek teeth. To examine the incisors, gently lift the lips for inspection. Be sure buccal surfaces are smooth (no horizontal ridges are present) and proper occlusion is met with mandibular incisors slightly caudal to the maxillary ones. A common noninvasive technique for examining the cheek teeth, which can be performed during every examination, is inserting an otoscope or vaginal speculum gently into the diasterna. Position the instrument caudally to visualize molars and premolars from both buccal and lingual surfaces (Figure 14-4). This technique does not provide the most thorough examination; however, it is relatively stress-free to the rabbit. If the rabbit is too stressed or uncooperative or a more thorough examination is necessary, lightly sedate the animal with an injectable sedative, or use isoflurane to get a better view. Once sedated, use a mouth speculum, mouth gag, or an endoscope to examine the molars (Figure 14-5). The most common locations for molar lesions are on the lingual surface of the mandibular cheek teeth and the buccal aspect of the maxillary teeth. The gingiva surrounding the molars should be at the level of the crowns except for the first mandibular cheek teeth in which the cranial aspect of the crowns will be more exposed. In addition to examining the teeth for overgrowth and malocclusion, it is important to inspect the oral mucosa and tongue for ulcerations and lacerations that may occur as a result of sharp points on teeth. The cheeks and jaws should be palpated for any swellings (e.g., abscesses) and the chin and dewlap examined for signs of drooling or slobbering. Abnormalities may be indicative of dental disease.
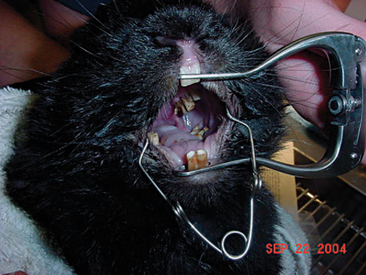
Figure 14-5 An oral examination performed under sedation enables the veterinarian to perform a more thorough exam.
The legs should be palpated from a proximal to distal position. Limb fractures are common sequelae to inappropriate handling. Rabbits do not have footpads. Closely examine the plantar surface of feet for pododermatitis. Pododermatitis is a common problem in rabbits housed in metal-grate caging.
DIAGNOSTIC TESTING
Clinical Pathology
Venipuncture should be done with care, as rabbit vessels are delicate and prone to hematoma formation. The maximum volume of blood that should be collected at one time is 1 ml/100 g body weight.9 Common sites for venipuncture include the jugular, lateral saphenous, cephalic and marginal ear veins. The jugular vein is the preferred site when a large sample is needed. To collect a jugular sample, the animal needs to be properly restrained. For restraint, a rabbit can be placed into a towel or a cat bag, and the neck gently extended in a manner similar to a cat; however, care should be taken not to extend the head too far. In some female rabbits, a large dewlap can reduce access to the jugular vein. Marginal ear veins can be used to collect blood samples with a 25- or 26-gauge needle. Generally, this site can only be used to collect small blood samples. This vein can also be used to place a catheter; however, catheterization can lead to vascular necrosis, thrombosis, and sloughing of the ear and should be avoided in breeds with small ears (e.g., dwarf breeds). The lateral saphenous vein is suitable for small- to moderate-sized blood samples (Figure 14-6). Placing alcohol over the venipuncture site or clipping the fur in that area can facilitate visualization of the vein. The cephalic vein can also be used to collect samples; however, this site is often reserved for intravenous catheter placement.
Hematology/Chemistry Panels
CBCs and serum chemistry panels should be performed in rabbits presenting for any disease process and as part of an annual exam. Rabbits have some unique hematologic differences in their CBC compared to other mammals. Healthy rabbits are primarily lymphocytic. Both small and large lymphocytes can be found in circulation. Rabbit lymphocytes are similar in appearance to this cell type in other vertebrates and are characterized by a deep blue cytoplasm, acentric nucleus, and high nuclear-to-cytoplasmic ratio. The acute inflammatory cell in rabbits is the heterophil. Although the function is similar to the neutrophil of other mammals, the staining characteristic of this cell type is different. Monocytes are the largest circulating white blood cell in rabbits and are characterized by diffuse nuclear chromatin, blue cytoplasm, and large, dark red granules in cytoplasm.9,11 Eosinophils have large granules and a bilobed or horseshoe-shaped nucleus. Low-grade eosinophilia can be seen with chronic parasitism. Basophils are relatively common in rabbits and can represent up to 30% of the differential in healthy animals. Infections rarely cause elevated white blood cell counts (>15,000 cells/ml) in rabbits; however, several changes may be associated with an acute infection, including a low total number of white blood cells (<5000 cells/ml) with a normal differential or normal cell counts with a shift to heterophilia, thrombocytopenia, and nucleated red blood cells. Other reasons nucleated red blood cells may be found are endothelial changes and regenerative responses.
There are no liver-specific enzymes in rabbits. In many mammals, alanine aminotransferase is liver specific; however, in rabbits, this enzyme is found in both the liver and cardiac muscles. Aspartate aminotransferase is found in a number of tissues, including muscle (cardiac, skeletal, smooth), liver, kidneys, and pancreas. Elevations of both enzymes, however, should be considered suspicious and other testing done to rule out liver disease (e.g., liver biopsy). (See Table 14-1 for rabbit hematologic and serum chemistry reference ranges.)
TABLE 14-1 Reference Hematologic and Serum Chemistry Values for Rabbits
| Parameter | Range | Comments |
|---|---|---|
| Erythrocytes (×106/μl) | 4.9-7.8 | Anisocytosis, polychromasia, low numbers of NRBCs, and Howell-Jolly bodies can be normal on rabbit blood smears for pet rabbits. |
| Hematocrit (%) | 31-50 | |
| Hemoglobin (g/dl) | 10-17.4 | |
| MCV (fl) | 57.5-75 | |
| MCH (pg) | 17.1-23.9 | |
| MCHC (%) | 28.2-37 | |
| Platelets (×103/μl) | 250-650 | |
| Leukocytes (×103/μl) | 5.2-12.5 | |
| Heterophils (%) | 20-75 | In healthy rabbits, heterophil-to-lymphocyte ratio is usually 1 : 1 or slightly lymphocytic. |
| Lymphocytes (%) | 30-85 | |
| Monocytes (%) | 2-10 | |
| Eosinophils (%) | 0-5 | |
| Basophils (%) | 0-8 | |
| ALP (U/L) | 4-16 | |
| ALT (U/L) | 14-80 | |
| AST (U/L) | 14-113 | |
| Bicarbonate (mEq/L) | 16.2-38 | |
| Bilirubin, total (mg/dl) | 0-0.75 | |
| BUN (mg/dl) | 13-30 | |
| Calcium (mg/dl) | 5.6-14 | |
| Chloride (mEq/L) | 92-112 | |
| Cholesterol (mg/dl) | 10-80 | |
| Creatinine (mg/dl) | 0.5-2.5 | |
| Glucose (g/dl) | 75-155+ | |
| LDH (U/L) | 43-129 | |
| Lipids, total (mg/dl) | 243-390 | |
| Phosphorous (mg/dl) | 2.3-6.9 | |
| Potassium (mEq/L) | 3.6-6.9 | |
| Protein, total (g/dl) | 5.4-8.3 | |
| Albumin (g/dl) | 2.4-4.6 | |
| Globulin (g/dl) | 1.5-2.8 | |
| Sodium (mEq/L) | 131-155 | |
| Triglycerides (mg/dl) | 124-156 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NRBCs, nucleated red blood cells.
Combined data from Carpenter JW, Mashima TY, Gentz EJ et al: Caring for rabbits: an overview and formulary, Vet Med Rabbit Symposium: Rabbits Are Not Small Cats, pp 348-380, April 1995; Harcourt-Brown F: Textbook of Rabbit Medicine, London, 2002, Elsevier; Mader DR: Basic approach to veterinary care. In Queensberry KE, Carpenter JW, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, St Louis, 2004, WB Saunders; Fudge AM: Laboratory Medicine: Avian and Exotic Pets, Philadelphia, 2000, WB Saunders.
The electrolytes reference ranges for rabbits are similar to those described for other mammalian vertebrates (see Table 14-1). Elevations in sodium and chloride can occur with dehydration or excess salts in the diet, whereas losses are generally associated with diarrhea or low dietary salt. Potassium levels may be low in animals with diets low in potassium or in animals that are starved. Total protein levels in rabbits can be higher compared to those levels found in other vertebrates.
Urinalysis
Urinary catheterization can be performed with a well-lubricated 9-French catheter inserted into the urethra.11 Rabbit urine is generally straw-colored and clear to cloudy. If the urine has a red appearance, then it is important to rule out blood in the urine from porphyrins. To differentiate hematuria from porphyrin pigmentation, use a urine dipstick or a woods lamp, as porphyrins will fluoresce. If a urinary tract infection is suspected, obtain a cystocentesis and submit a culture and sensitivity in addition to performing a complete urinalysis. Cloudy urine in rabbits is usually due to calcium excretion and the presence of crystals. The presence of crystals in the urine can make it difficult to identify bacteria on a cytologic test, and a culture should be performed if a bacterial cystitis is suspected. (See Box 14-2 for normal urinalysis values.)
BOX 14-2 Reference Ranges for a Rabbit Urinalysis
From Mader DR: Basic approach to veterinary care. In Queensberry KE, Carpenter JW, editors: Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, St Louis, 2004, WB Saunders.
| Specific gravity | 1.003-1.036 |
| pH | 8.2 |
| Crystals | Calcium carbonate monohydrate, anhydrous calcium carbonate, ammonium magnesium phosphate |
| Casts, cells, bacteria | None |
| WBC | Occasional |
| RBC | Occasional |
| Albumin | In young occasionally |
| Average urine production | 130 ml/kg/day |
Diagnostic Imaging
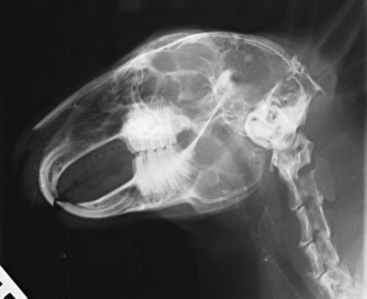
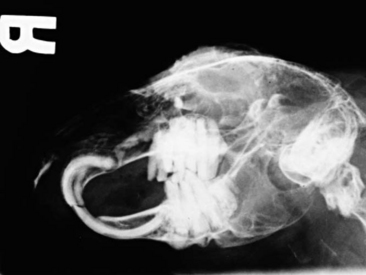
Radiography is a useful diagnostic test in rabbit medicine. It is important to always take at least two radiographs (e.g., lateral and dorsoventral/ventrodorsal). Many times radiographs of the abdomen or thorax can be taken with little or no sedation if the handlers are patient and careful. If more restraint is necessary, isoflurane or midazolam can be used to sedate or immobilize the patient. Skull radiographs are required to evaluate the roots of molars and incisors and should be taken in cases of suspected or known malocclusions. The most useful radiographic views for examining the teeth are rostrocaudal, dorsoventral, and lateral (Figure 14-7). In cases of ocular discharge, contrast media can be injected into the lacrimal duct to determine if any of the molar roots are impinging on the duct (Figure 14-8). Chronic cases of otitis or neurologic disease warrant evaluation of the tympanic bullae for radiographic changes. In cases of facial abscess, radiology can be used as a prognostic indicator; periosteal bone reaction decreases the prognosis for full recovery.
Abdominal radiographs can be used to assess the gastrointestinal, reproductive, and excretory systems. Survey radio graphs can be useful for diagnosing ileus or gastric foreign bodies. Rabbits with severe generalized ileus carry a grave prognosis. Rabbits with trichobezoars are frequently anorectic. Therefore, the presence of a soft tissue mass in the stomach of an anorectic rabbit is highly suggestive of the presence of a gastric foreign body (e.g., trichobezoar). In cases where a gastrointestinal foreign body is suspected but not conclusive from survey radiographs, the veterinarian should consider doing a contrast study. Uterine adenocarcinoma should be considered in a differential list for any intact doe with a suspicious soft tissue mass in the caudoventral abdomen. Renal, urethral, and cystic calculi can generally be diagnosed from survey radiographs, although contrast studies are also needed in some cases.
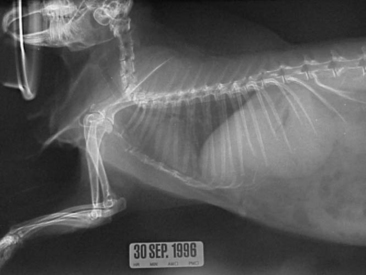
Thoracic radiography is an important diagnostic test for ruling out respiratory and cardiac disease. The thoracic field of a rabbit is small in comparison with that of other mammalian vertebrates (Figure 14-9). However, radiograph interpretation is similar. Pneumonia, metastatic neoplasia, and trauma are common disease problems that can be diagnosed radiographically.
In addition to radiography, the other commonly used diagnostic imaging techniques can also be useful for assessing the rabbit patient. Ultrasound can provide excellent images of the abdomen and thorax and has been used to diagnose abdominal pathology, uterine disease, foreign bodies, excretory calculi, and cardiac disease. Success with ultrasound is based on the sonographer having a good knowledge of anatomy, so that he or she can interpret an image based on the position of the probe. A 7.5- to 10-mHz transducer is recommended for rabbit ultrasonography. Clipping the fur of the rabbit in the area of interest can also help improve the image by providing the contact between the skin and the probe; however, caution must be used when clipping the fur, as the skin tears easily. Echocardiography is an appropriate diagnostic aid in cases of suspected heart disease or in patients with audible murmurs. Echocardiographs should be conducted using a high-frequency transducer (7.5-12 MHz) and high frame rate ultrasound machine.4 Magnetic resonance imaging and computed tomography (CT) can prove invaluable in diagnosing certain conditions, including diseases associated with the sinuses, inner ear, cranium, and teeth. Although these tests are becoming more available, they still remain cost prohibitive for some clients.
Microbiology
Rabbits can become bacteremic and develop an infection in practically any tissue. The most common sites of infection, however, are the urinary bladder, respiratory system, and integument. Urine cultures obtained from cystocentesis are preferred to those collected via free-catch. Rabbit urine should have a basic pH. If the urine pH becomes acidic, it will promote the growth of potential opportunistic pathogens. Most pathogens of the urinary tract are Gram-negative bacteria. Female rabbits are more susceptible because of their stouter urethras. In addition to urine cultures, deep nasal cultures for chronic respiratory disease and cultures of abscesses are considered routine. Deep nasal cultures usually require sedation, as the microculturette is advanced into the nasal passage up to the level of the medial canthus of the eye. When culturing an abscess, the best results are often obtained from sampling the abscess wall.11 Samples collected from the nasal sinuses and abscesses often have a significant number of inflammatory cells associated with them. These cells can affect the results of a culture by reducing the number of organisms to the point where analytical sensitivity of culture cannot detect the bacteria. In some cases, serial samples may be needed to determine if an organism is a trace pathogen. Culturing microorganisms from rabbits is otherwise routine. If anaerobes are suspected, then the veterinarian should contact the laboratory to obtain the appropriate media for collection and transport.
Parasitology
Although rabbits are captive bred, and the expectation is that parasites would be absent or low in these animals, parasites remain a problem because few preventive health programs are instituted at the breeding population level. Endoparasites can be diagnosed using routine direct saline smears or fecal flotations. Direct saline smears are preferred for identifying protozoa, whereas a fecal flotation is preferred for finding nematodes, trematodes, and cestodes. Annual parasitological examinations include both techniques. Because parasites can be shed transiently, serial samples should be performed. Pooling fecal samples produced over the course of a week may reduce the likelihood of misclassifying a rabbit’s parasitic status. For systemic parasitologic disease, serologic testing or histopathology is required. E. cuniculi, a microsporidian, is generally diagnosed antemortem via serology or postmortem via histopathology. E. cuniculi is shed in the urine and may also be diagnosed from direct visualization in a urine sample, but this is not analytically sensitive. Eimeria steidae, a hepatic coccidian, can be diagnosed antemortem via a fecal direct smear or postmortem via histopathology.