CHAPTER 55 Pythiosis and Zygomycosis
Although the oomycete Pythium insidiosum and the zygomycetes Conidiobolus spp. and Basidiobolus ranarum fall into two taxonomically distant groups of organisms, they are typically grouped together by equine clinicians and pathologists because they share similar clinical and histologic characteristics. Both cause cutaneous lesions characterized by pyogranulomatous and eosinophilic inflammation associated with broad, sparsely septate hyphae. Because of these similarities, pythiosis and zygomycosis have been referred to collectively as “phycomycosis.” Despite remaining a convenient label for cases without a definitive, culture-based diagnosis, “phycomycosis” is no longer an appropriate taxonomic designation and should be replaced in current literature with the more specific terms pythiosis and zygomycosis. Clinically, differentiating between these infections is important because of differences in epidemiology, choice of therapy, and prognosis.
PYTHIOSIS
Pythiosis, a pseudofungal infection caused by the oomycotic pathogen Pythium insidiosum, is best known as a cause of cutaneous and subcutaneous disease in horses1 and of gastrointestinal or cutaneous disease in dogs.2 It has also been described as an uncommon cause of cutaneous and subcutaneous lesions in cats and calves3,4 and of arteritis, keratitis, or periorbital cellulitis in humans.5,6 In older literature, P. insidiosum has been referred to using the now-outdated synonyms Hyphomyces destruens,7 Pythium destruens,8 Pythium spp.,9 and Pythium gracile.10
Clinical manifestations of pythiosis have been recognized in horses for more than a century and variably referred to as “kunkers,” “Florida horse leeches,” “bursatti,” and “swamp cancer,” as well as “phycomycosis.” The disease was first noted in the mid-nineteenth century when British veterinarians working with horses in India observed a chronic granulomatous cutaneous disease that they termed “bursautee.” A fungal etiology for this disease was suspected on the basis of histologic findings in the late nineteenth century,11 and the pathogen was isolated as early as 1901 by Dutch investigators working with horses in Indonesia.12 However, it could not be induced to sporulate using standard fungal media and was thus assumed to be a sterile zygomycete or “phycomycete” fungus based on the morphologic characteristics of its vegetative hyphae. It was not until 1974 that Austwick and Copland13 were able to produce biflagellate zoospores from isolates obtained from horses in New Guinea, identifying the pathogen as an oomycete that was likely a member of the genus Pythium. The species name Pythium insidiosum was introduced in 1987 by de Cock,14 who was able to produce sexual reproductive structures from pathogenic isolates, and who found the morphologic characteristics of several isolates from horses and dogs to be identical.
Etiology and Epidemiology
The causative agent of pythiosis is the aquatic oomycete Pythium insidiosum, a member of the kingdom Stramenopila that is more closely related to algae than to true fungi.15 The taxonomic differences between oomycetes and fungi are reflected on the cellular level by differences in cell membrane and cell wall composition. Ergosterol, an essential component of the fungal cell membrane and a common target of antifungal drugs, is not a principal sterol in the oomycete cell membrane.16 In addition, oomycetes generally lack chitin, which is an important structural component of the fungal cell well.
The infective stage of P. insidiosum is thought to be the biflagellate zoospore, which is released into warm-water environments and swims in a helical pattern as part of a complex homing sequence that allows it to locate, move toward, and encyst on specific host tissues.17 P. insidiosum zoospores are attracted to animal hair, as well as to cut edges of skin, and likely cause infection by encysting in damaged skin or gastrointestinal (GI) mucosa.18 Although many infected horses have a history of recurrent exposure to standing fresh water,19,20 other risk factors for the development of pythiosis have not been identified. Affected animals are immunocompetent and otherwise healthy. Given the affinity of P. insidiosum zoospores for damaged skin, it seems likely that animals with cutaneous wounds or parasite-induced injury to GI mucosa would be more likely to become infected. However, documented epidemiologic evidence to support this assumption is lacking. The presence of a traumatic wound before the development of cutaneous pythiosis has been reported in a small number of canine and equine cases. Because traumatic incidents are rarely observed by the owner, however, it is often difficult to determine whether lesions noted early in the course of disease resulted from trauma or from early infection.
Clinical Findings
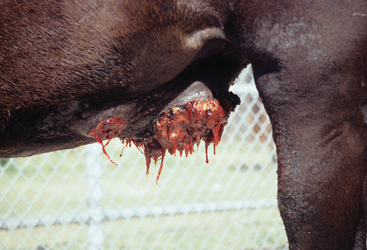
Pythium insidiosum infection in horses most often causes large, ulcerative, proliferative granulomatous lesions involving cutaneous or subcutaneous tissues of the distal limbs (generally below the knee and hock), ventral abdomen (Fig. 55-1), ventral thorax, or face.1,7,21,22 Less frequently affected areas include the dorsum and external genitalia.20 Solitary lesions are most common,21 but multiple lesions have been reported.7,20,21,23 Intense pruritus is typically associated with cutaneous pythiosis and often results in self-mutilation of the affected tissues. Lesions typically appear as circular masses that are rapidly expanding, ulcerative, and necrotic and that contain multiple fistulous tracts that drain a serosanguineous, hemorrhagic, or mucopurulent fluid.1 In addition, a characteristically stringy, viscous fluid may be observed hanging in strands from ventral abdominal lesions (Fig. 55-1) or matting the hair around extremity lesions.
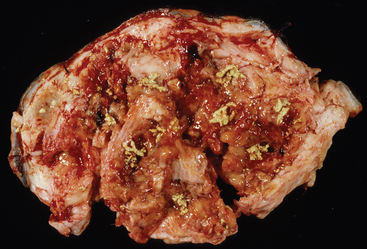
Sinus tracts in P. insidiosum lesions contain multiple, 1-mm to 10-mm, tan to yellow, branching, coral-like firm masses or coagula that are commonly referred to as “kunkers” or “leeches” (Fig. 55-2). Histologically, these kunkers are composed of hyphae, inflammatory cells (especially eosinophils), collagen, and necrotic debris, and they are especially prevalent deep to the junction of the ulcerated portion of the lesion and the intact epidermis.24 Kunkers are often extruded from draining tracts and may be found in bandage material. Other diseases that can cause similar cutaneous lesions in horses include cutaneous habronemiasis, excessive granulation tissue, bacterial granulomas, sarcoid, squamous cell carcinoma, and zygomycosis. Both habronemiasis and zygomycosis can produce tissue grains, but in habronemiasis they are usually smaller and lack the typical coral-like shape of kunkers associated with pythiosis.24,25
Although most P. insidiosum lesions are confined to cutaneous and subcutaneous tissues, long-standing infections may sometimes invade deeper tissues. Extension of pythiosis through fascial planes resulting in infection or inflammation of an underlying tendon sheath, joint, or bone has been described in horses with extremity lesions present for longer than 2 months.23,26,27 Horses with bone involvement are often presented with lameness (which may be non–weight bearing) and edema of the affected limb. Radiographic findings are characterized by extensive, disorganized bony proliferation with variable osteolysis and cortical erosion.26–28 Bones in which infection has been observed include the phalanges, the third metacarpal and metatarsal bones, and the proximal sesamoid bones. Periostitis resulting from adjacent soft tissue infection may cause significant periosteal proliferation without actual invasion of the periosteum; therefore, in cases with radiographic changes that are limited to proliferative changes of the periosteum, differentiation between periostitis and osteomyelitis cannot be made without a biopsy.28
In addition to local invasion of deeper tissues, dissemination of chronic cutaneous P. insidiosum infection to regional lymph nodes and lung has been reported in a small number of horses. Infection of an inguinal lymph node has previously been described in four horses with cutaneous hindlimb lesions.24,29 Likewise, pulmonary lesions have been described in three horses with preexisting cutaneous lesions.1,30,31 In one of these cases, however, the organism was not isolated, and the gross and microscopic characteristics of the lesions were not described.31
Intestinal granulomas similar to the enteric lesions found in dogs with GI pythiosis have been described in four horses.32–35 Clinical signs in these horses included colic, and in each case a jejunal mass was detected during abdominal exploratory (three horses) or necropsy (one foal). Two of these horses had no recurrence of pythiosis after resection of the jejunal lesion; a third horse was euthanized at surgery because of extensive jejunal infarction thought to have resulted from prolonged intestinal distention.35
Diagnosis
Culture
Isolation of P. insidiosum from infected tissues is not difficult when appropriate sample-handling and culture techniques are employed. However, because these techniques are fairly specific, it is important to use a laboratory with expertise in the isolation of pathogenic oomcyetes. Kunkers are more likely than tissues to provide a positive culture and are the preferred source of inoculum.36 For best results, unrefrigerated kunkers should be wrapped in a sterile, saline-moistened gauze sponge and shipped at ambient temperature to arrive at the laboratory within 24 hours of collection. However, when samples cannot be processed for more than 2 to 3 days after collection, they should be shipped with ice packs, stored in the refrigerator, or stored at ambient temperature in an antibiotic solution to decrease proliferation of bacterial contaminants.36
The use of selective media significantly increases the likelihood of isolating pathogenic oomycetes, especially from lesions with secondary bacterial infection. The author routinely uses vegetable extract agar37 amended with streptomycin (200 μg/mL) and ampicillin (100 μg/mL) for the isolation of P. insidiosum. As a commercially available alternative, Campy blood agar (Remel, Lenexa, Kansas), which contains trimethoprim, vancomycin, polymyxin B, cephalothin, and amphotericin B, is also effective. Small pieces of fresh kunkers should be placed directly on the surface of the agar and incubated at 37° C (98.6° F); growth is typically observed within 24 hours.
Although the identification of oomycetes is generally based on morphologic features of sexual reproductive structures such as oogonia and antheridia, isolates of P. insidiosum rarely produce these structures in vitro. Therefore, identification of P. insidiosum should be based on colonial and hyphal characteristics; growth at 37° C; production of motile, reniform, biflagellate zoospores; and, if possible, specific polymerase chain reaction (PCR) amplification or ribosomal ribonucleic acid (rRNA) gene sequencing. Colonies on vegetable extract or Sabouraud dextrose agar are typically submerged, white to colorless, and have an irregular radiate pattern.14,38 Microscopically, hyphae are broad (4-10 μm in diameter), hyaline, sparsely septate, and tend to branch at right angles. Zoospores can be readily produced by placing boiled grass blades on the surface of a 1- to 2-day-old colony growing on 2% water agar, incubating at 37° C for 18 to 24 hours, and then placing the infected grass blades in a dilute salt solution.39–41 After 2 to 4 hours of incubation at 37° C, terminal vesicles from which zoospores are released can be visualized extending from the cut edges of the infected grass blades. Although the production of zoospores is an important supporting feature for the identification of pathogenic oomycetes, it is not specific for P. insidiosum. It does, however, rule out zygomycosis.
Serology
Both immunoblot42 and enzyme-linked immunosorbent assay (ELISA)43,44 have been used successfully to demonstrate the ability of sera from Pythium-infected horses to recognize soluble mycelial antigens of P. insidiosum. Unfortunately, the only assay currently available to practicing veterinarians has not been tested for specificity using serum from horses with basidiobolomycosis or sporotrichosis, or from healthy horses that are regularly exposed to nonpathogenic oomycetes in pasture environments; therefore its rate of false-positive results is unknown. In the author’s experience, the specificity of immunoblot serology for the diagnosis of pythiosis in horses is not as high as in dogs and cats; occasional false-positive results are observed in horses with other types of fungal as well as nonfungal inflammatory diseases.
Molecular Assays
To circumvent the difficulties associated with obtaining a culture-based diagnosis of pythiosis, a P. insidiosum–specific PCR assay was recently developed.45 This assay can be applied to deoxyribonucleic acid (DNA) extracted from either cultured isolates or appropriately preserved, infected tissue samples.46 In addition, the author has successfully applied this technique to DNA extracted from paraffin-embedded tissue sections.47 The major advantage of this assay is its high specificity.
Immunohistochemistry
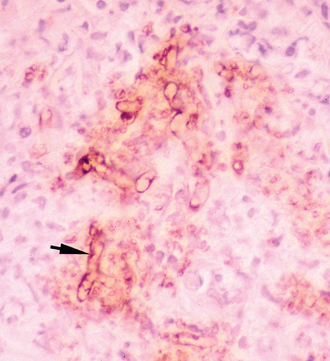
Immunohistochemical (IHC) techniques using polyclonal antibodies, developed first by Brown et al.48 and later by Patton et al.,49 have previously been used as confirmatory tests for pythiosis. These techniques have the advantage of being applicable to paraffin-embedded tissues. However, at least one of these antibodies has demonstrated cross-reactive staining of Conidiobolus and Lagenidium hyphae in canine tissue.50,51 Therefore the specificity of this antibody for the IHC diagnosis of pythiosis is questionable. A new polyclonal anti–P. insidiosum antibody raised in chickens and adsorbed with sonicated Lagenidium and Conidiobolus hyphae appears to be highly specific for the IHC detection of P. insidiosum hyphae in both equine tissues (Fig. 55-3) and canine tissues,52 but it is not commercially available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree