CHAPTER 75 Puerperal Nutrition and Metabolic Diseases
The time around parturition is the single most stressful event in a doe’s reproductive life cycle. Metabolic and nutritional insults before and following parturition can result in exacerbation of key metabolic changes necessary to make the physiologic transition from pregnancy to lactation, resulting in a myriad of metabolic diseases. Current perceptions tend to focus attention on the lactating doe and nutritional support of milk production; however, nutrition and management of the late pregnant doe has more significant impact on her productive efficiency, both reproductive and lactational. The focus of this chapter will be to highlight the impact of prepartum nutrition and management on periparturient metabolic disease and reproduction.
METABOLIC ADAPTATIONS AROUND KIDDING
An appreciation of the exquisite metabolic adaptations the doe must undergo to achieve a successful transition from pregnancy into lactation is key to understanding the critical role of nutrition on metabolic disease and reproductive performance. Minimal data are available regarding pregnant doe metabolism and nutrition. Given the similarity in metabolic responses observed with dairy cattle and sheep, current research concepts regarding physiologic alterations associated with the transition from pregnancy to lactation will be extrapolated from these species to the pregnant doe. Fetal metabolism and maternal metabolic adaptations over the periparturient period were detailed in Chapter 58. Important concepts as they relate to induction of metabolic disease or influencing reproductive performance are summarized here.
Fetal Metabolism
Metabolic activity of the fetus is nearly twice that of the dam, and fetus, placenta, and uterus combined (conceptus) consume nearly 50% and 80% of maternal glucose and amino acid supply, respectively.1 The conceptus oxidizes glucose and its placental derivative, lactate, as its primary metabolic fuel; however, glucose and lactate oxidation only account for 50% to 60% of fetal caloric needs.2 Amino acids, even in a well-fed dam, account for 30% to 40% of total conceptus caloric requirement.2 Fetal utilization of glucose is dependent upon maternal glucose availability and with maternal undernutrition, utilization is markedly decreased. Amino acid delivery to the conceptus is independent of maternal dietary adequacy, and thus, amino acids become a primary fetal energy substance when glucose is unavailable.3 Placental active transport and catabolism of maternal skeletal protein account for the ability of the dam to maintain consistent amino acid delivery to the conceptus.1,2 In spite of their ready availability in maternal circulation, nonesterified fatty acids (NEFAs) and ketone bodies are not significant energy substrates for the gravid uterus as they do not efficiently cross the placenta.2 This metabolic milieu provides a buffering system to maintain fetal growth in the face of inadequate maternal nutrition, yet not facilitate excessive fetal growth at the expense of maternal energy reserves (fat).
All macro- and microminerals are efficiently transferred from maternal circulation to the conceptus. Within the fetus, minerals are concentrated in the fetal liver as well as being utilized to meet their specific metabolic or structural physiologic roles.4 Concentration of mineral elements within the fetal liver provides a mineral reserve in support of the neonate’s higher metabolic rate and in the face of consuming a trace mineral–deficient diet (e.g., milk). Fat-soluble vitamins A, D, and E do not efficiently cross the placenta, but are concentrated in colostrum.5–7 With the transfer of minerals and vitamins from maternal circulation to fetus and colostrum, adequacy of the late gestation maternal diet will ultimately dictate nutrient status of dam and neonate and mediate disease susceptibility potential, especially susceptibility to infectious diseases, given the important role trace minerals and fat-soluble vitamins play in immune function.
Maternal Adaptations
The goal of maternal metabolic adaptations is to increase glucose availability in support of pregnancy or subsequently lactation. To meet glucose needs during the transition from late pregnancy to lactation, maternal tissues reduce their use of glucose as an energy source, increase rate of hepatic gluconeogenesis, and provide sufficient endogenous glucogenic substrate (i.e., amino acids, glycerol, lactate) to account for lower nutrient intake.8 Glucose is replaced by fatty acids and their derivative, ketone bodies, as alternative maternal metabolic fuels. To facilitate fatty acid availability, adipose tissue becomes sensitized to signals mediating reduced lipogenesis and increased lipolysis, hence increasing blood NEFA concentration. Reduced glucose uptake and increased NEFA oxidation by skeletal muscle accounts for a significant savings of glucose; however, skeletal muscle also contributes amino acids in support of gluconeogenesis. Coordinated adaptation of adipose tissue, skeletal muscle, and liver function is mediated through changes in insulin concentration and tissue insulin resistance.8
Elevation of blood NEFA concentration metabolically is a double-edged sword. As described, elevation of blood NEFA in late pregnancy provides an alternative fuel for maternal nonuterine tissues. However, the liver absorbs NEFAs in direct proportion to their blood concentration. Hepatocytes have limited ability to completely oxidize influx of NEFAs, and they are partially oxidized into ketone bodies, which can be metabolized for energy by other nonuterine maternal tissues.9 Available NEFAs can also be re-esterified into triacylglycerol (TAG) and packaged into very low density lipoprotein (VLDL) particles and exported from the liver.9 If not exported in VLDL, then TAG is packaged into hepatocytes, resulting in increasing amounts of fatty infiltration. Ruminant animals in general are not efficient at exporting VLDL particles10 and thus experience variable degrees of hepatic fatty infiltration around the time of parturition.9
Similar to cattle and sheep, blood NEFA concentration in goats is a metabolic marker of energy balance and degree of fat mobilization.11 Excessive elevation of blood NEFA concentration can result from factors inducing a stress response (β-adrenergic agents stimulate lipolysis), prolonged or severe negative energy balance, or both. Dry matter intake typically declines just before, and slowly increases following parturition, placing the animal in a state of negative energy balance. Errors in feeding management, poor quality feeds, adverse environmental conditions, and number of fetuses, among other factors, can exacerbate severity and duration of negative energy balance. Hepatic uptake of blood NEFA beyond its metabolic capacity to process will result in maladaptive responses leading to ketosis and increasing fatty infiltration, which compromise hepatic function and initiate a deteriorating metabolic cycle of hypoglycemia, fat mobilization, and severe fatty infiltration.
Although hypocalcemia is not as prevalent in goats compared to dairy cattle, the doe’s calcium homeostatic system must adapt to the challenges of calcium outflows associated with fetal growth and initiation of lactation. Limited studies have specifically addressed regulation of calcium metabolism in the goat, but evidence suggests similar controlling mechanisms compared to cattle.12 Maternal adaptations to trace mineral and fat-soluble vitamin losses to fetus(es) and colostrum are limited to mobilization of reserves and replenishment from adequate dietary supplementation.
PREPARTUM NUTRITION AND REPRODUCTION
Nutrient requirements of the late pregnant, nonlactating doe are only slightly higher than maintenance, approximately equivalent to the energy and protein required to produce 2 to 4 lb of 4% milk per day.13 However, providing sufficient quantities of essential nutrients is just as critical for the late pregnant doe as the lactating doe to maintain optimal performance. Recognition of a substantial increase in nutrient requirements for the late pregnant and lactating animal compared to maintenance has been the focus of nutritional investigations in dairy cattle and ewes. Dietary recommendations for digestible energy (DE), crude protein (CP), calcium (Ca), and phosphorus (P) for the late gestation doe are 1.5 to 1.8 times greater compared to maintenance at the same level of activity.13 The transition from late gestation into lactation requires a similar or much greater increase in dietary nutrient intake. These differences in nutrient requirements require appropriate modifications in the feeding program as well as metabolic alterations by the doe to adequately support late gestation and lactation. If these metabolic changes are not effectively enacted, metabolic disease, reduced milk production, and impaired reproductive performance may result.
Kid Viability and Survival
Data from cattle and sheep suggest that nutrition of the dam at all stages of gestation can influence neonate viability and productivity. In reviewing factors responsible for contributing to prepartum and partum calf14 or lamb15,16 losses, nutritional deficiencies and toxicities influenced all factors. Similar contributing factors can be reasonably assumed for goats. Maternal undernutrition during mid- to late gestation has been implicated in an abortion syndrome observed in yearling Angora goats.17
Birth weight is the single most important factor determining postnatal survival. Extremely heavy birth weight is more associated with dystocia, and lighter birth weight kids, typical of twins and triplets, have higher mortality rates.18 Dynamic in vivo measures of fetal sheep crown-to-rump length found fetal growth to be deterred or completely stopped during periods of induced maternal hypoglycemia during late pregnancy.19 Ditocous ewes fed an 8% CP diet gave birth to lambs that were 20% lighter than lambs born to similar ewes fed isocaloric diets with either 11% or 15% CP.20 Ewes fed 11% CP diets, however, mobilized maternal protein in support of fetal growth, whereas maternal protein accretion occurred in ewes fed 15% CP diets. In contrast, singleton-bearing ewes fed 1.4 times their estimated protein requirement (165 g CP; 11.8% CP) delivered larger lambs (4.9 versus 4.3 kg) with greater lambing difficulty and higher mortality rate compared to ewes fed to requirement (117 g CP; 8.7% CP).21 Besides differing in using twin or singleton pregnant ewes, dietary treatments were initiated at 110 days20 and 85 days21 of gestation for these two studies.
Maternal dietary influence on fetal growth is more complicated than simply addressing under- or overfeeding relative to requirement. Maternal body condition score and dietary nutrient status relative to period of fetal and placental growth are confounding variables.1 Fat ewes partition more nutrients to the gravid uterus, maintaining fetal growth during periods of moderate undernutrition in late pregnancy compared to lean ewes.22 Lean or moderately fat ewes fed ad libitum in late pregnancy had similar placental and fetal birth weights despite different intake amounts (29% higher for lean ewes), suggesting placental mitigation of available nutrients in controlling fetal growth.23 West African dwarf does fed a higher level of concentrate throughout gestation had heavier birth weight kids and greater problems with dystocia, whereas does fed the same rate of concentrate for only the third trimester (>120 days) had low birth weight kids with higher mortality rate.24 Does fed a higher and moderate amount of concentrates in second (60 to 120 days) and third trimester, respectively, had moderate birth weights and minimal health problems. High rate of energy supplementation during first and third trimester in does was suggested to be avoided.25 Twin-bearing Tswana does supplemented throughout pregnancy had greater total kid birth weight compared to does not supplemented or supplemented before or after 103 days of gestation.26 Although placental mass was not influenced by dietary treatment, nonsupplemented does had the lowest placental (390 versus 580 g) and total fetal (5.1 versus 5.8 kg) weights.26
In primigravid, singleton-bearing ewes, placental growth and, ultimately, lamb birth weight were restricted when fed for rapid growth after the first trimester.27 Rapid maternal growth during the first trimester followed by moderate growth stimulated compensatory placental growth and moderate birth weight lamb.27 Placental weight is the primary determinant of fetal weight.28 Fetal cotyledon number was influenced by first trimester nutritional status, whereas cotyledon weight was mediated by second trimester nutrition.27 Fetal number and placement within uterine horns further mitigate the relationship between gestational nutrition and fetal growth.28
Beyond birth weight, maternal milk production will affect growth and survival of the neonate. Inadequate nutrition during late pregnancy influences milk production and composition,29 possibly as a result of compromised mammary gland development.30 Dietary protein content of 11% CP (9.8 g/kg body weight × 0.75), slightly higher than the National Research Council (NRC) recommendations,13 is recommended for adequate late gestation nutrition to meet fetal and subsequent lactational needs.31 Current NRC pregnancy energy requirements were considered adequate;29 however, a new NRC publication for goat nutrient requirements is under development.
Colostrum Quality and Quantity
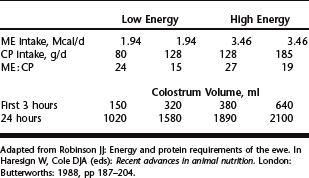
Following birth weight concerns, adequate consumption of good quality colostrum is essential to neonatal survival. With selection for higher milk production in dairy goat breeds, potential exists for selecting against colostrum quality, as a result of increased colostrum volume, similar to what has been experienced in the dairy industry. Limited studies, with equivocal results, have addressed nutritional effects on colostrum quality in sheep and goats. Contrary to what might be expected, feeding greater amounts of prepartum protein (117 versus 165 g/day) resulted in reduced colostrum yield in sheep, but immunoglobulin concentration was not reported.21 Energy and protein content of the prepartum diet for pregnant ewes influenced the amount of colostrum produced at 3 and 24 hours after lambing.32 Colostrum volume increased with increasing supply of energy and protein (Table 75-1). In ewes, increasing prepartum energy intake increased colostrum yield, and increasing prepartum protein intake improved lamb colostral immunoglobin absorption efficiency.33 Although feeding supplemental concentrate increased colostrum yield, immunoglobulin concentration was decreased even with increasing undegradable protein supplementation.34 It would seem that beyond severe undernutrition, colostrum quality is not greatly influenced by additional energy or protein supplementation and may adversely affect quality by increasing volume. Further work is needed to fully explore prepartum diet effects on colostrum production and quality.
Fetal Reproductive Development
Adequacy of the prepartum diet has been shown to influence embryonic and fetal ovarian development and subsequent reproductive performance in the adult ewe.35 Gunn and colleagues36 observed a reduction in lambing percentage from ewe lambs born to ewes exposed to undernutrition during their fetal development compared to ewe lambs born to well-fed pregnant ewes. Increased numbers of high-quality embryos were collected from lambs born to ewes fed a higher level of nutrition (1.5 times maintenance) during mid-gestation (71–100 days), late gestation (101–126 days), or both.37 Whether this nutritional effect is influencing ovarian growth, follicular development, or embryo survival is not well defined, but given the lifetime ramifications, further study to elucidate mechanisms is warranted.
Periparturient Disease
Unlike the situation for cattle given the difference in time frame between parturition and subsequent mating, periparturient disease of goats plays a lesser role in mitigating reproductive performance than recognized for dairy cattle. If the periparturient disease process induces severe body condition loss and an inability to regain condition before breeding, then reproductive performance may be adversely affected. The most significant reproductive effect of periparturient disease is reduced survival of offspring and dam rather than specific impact on subsequent reproductive efficiency. Role of prepartum nutrition in the pathogenesis of periparturient diseases is discussed in the following sections.
DERANGEMENTS OF ENERGY BALANCE (PREGNANCY TOXEMIA, KETOSIS, AND FATTY LIVER SYNDROME)
An inability of the pregnant or lactating doe to maintain glucose homeostasis characterizes a state of negative energy balance whereby glucogenic precursors from diet or endogenous sources are insufficient to meet the doe’s total energy requirement. Metabolic signals recognizing glucose insufficiency initiate fat mobilization in proportion to degree of negative energy balance to compensate. If magnitude or duration of the negative energy balance insult is severe enough, release of NEFA may overwhelm the liver’s capacity, culminating in maladaptive metabolic responses generating large quantities of ketone bodies and allowing severe fatty infiltration of hepatocytes. Ability to maintain functional insulin concentration and maternal tissue responsiveness seems pivotal to appropriate metabolic adaptation to negative energy balance.9,38 Inherent metabolic differences among individuals (genetic variation) may account for observed disparity in individual susceptibility to clinical manifestations.39
Any animal, management, or environmental factors that adversely affect the doe’s net energy intake increases the risk of metabolic maladaptation leading to ketosis and fatty liver infiltration. Overconditioned does (>4.0 body condition score [1 to 5 scale]) are at high risk for ketosis and fatty liver as they experience reduced feed intake compared to leaner does and have abundant fat reserves to mobilize. Feed availability and quality, sudden feed changes, water availability, transportation of late gravid does (>100 days), concurrent disease, and heat or cold stress conditions are some of the factors that, singly or in combination, may initiate an episode of reduced feed intake potentially leading to a ketotic response.39,40 Pregnancy toxemia or lactational ketosis induced by a concurrent disease is often categorized as secondary ketosis, as typically the underlying disease was responsible for reduced intake and inducing the ketotic state.
Most often, pregnancy toxemia or lactational ketosis is a sporadic occurrence of low morbidity (<3%) within a goat herd. Individual variation in the metabolic response to herd nutrition and management accounts for the sporadic nature of the disease. In contrast, factors influencing a large percentage of the herd, namely nutritional and feeding management factors, can result in a herd outbreak with morbidity rates exceeding 10% of the does. Timely recognition of individual cases and underlying etiology are important in heading off potential herd problems as mortality rates often exceed 80% in affected animals.39
Clinical Signs and Diagnosis
Clinical presentation of an affected doe will vary with associated metabolic derangements. Initially with pregnancy toxemia clinical signs will be vague, primarily behavioral and attitude changes. Does will generally isolate themselves and appear listless, dull, and depressed. As hypoglycemia and ketonemia progress, neurologic manifestations become evident. Does may appear blind, become disoriented and ataxic, and have reduced feed intake. Metabolic acidosis develops from excessive ketone body production. Breathing may become more rapid, neurologic signs continue to progress, and the doe is less likely to rise or respond to stimuli. Chewing, teeth grinding, and vigorous licking movements may be seen. In the final stages the doe becomes recumbent with severe neurologic signs and coma. Death is usually the result of renal failure or toxemia following fetal death. Does may show slight signs of recovery following fetal death but may ultimately succumb as a result of toxin release. Progression of clinical signs ranges from 12 hours to 7 days, a more typical time course being 3 to 4 days.39,40 Differential diagnoses should include hypocalcemia, polioencephalomalacia, listeriosis, and ruminal acidosis (grain overload).
Ketosis is definitively diagnosed by identifying excessive positive ketone reaction in urine. Dipsticks and ketone powder both use the nitroprusside test to semiquantify acetoacetate concentration. Serum or plasma β-hydroxybutyrate (BHB) concentrations can also be determined for diagnosis. Expected BHB concentration is below 8 mg/dl (0.76 mmol/L), whereas concentrations above 15 mg/dl (1.4 mmol/L) indicate moderate ketogenesis and subclinical disease. Clinical ketosis is often associated with BHB concentrations in excess of 25 mg/dl (2.4 mmol/L) and may exceed 40 mg/dl (3.8 mmol/L). Other laboratory findings suggestive of ketosis include elevated NEFA (>0.4 mEq/L) and hypoglycemia (<30 mg/dl). Depending upon the degree of associated fatty liver, elevation of some liver enzymes and lower total cholesterol concentration may be present. Degree of fatty liver infiltration is most often diagnosed on necropsy. In the later stage of the disease, hyperglycemia, hypokalemia, hypocalcemia, and elevated creatinine and urea nitrogen may be evident. Hyperglycemia may occur following fetal death. Anorexia may account for hypokalemia and hypocalcemia. Associated dehydration results in elevated creatinine and urea nitrogen concentrations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree