Poultry may be purchased from any commercial supplier. Poultry breeding companies in the UK will supply fertilised eggs or day-old chicks. Poultry flocks may have high health status or be of conventional status with unknown pathogen load. Researchers should always establish the health and vaccinal status of the supply flock.
By law, all poultry premises with more than 50 birds are required to register with Department for Environment, Food and Rural Affairs (DEFRA). Registration can be carried out on-line at http://poultry.defra.gov.uk.
Biology
External features
There is a keratinised beak, without lips or teeth. The legs are covered in scales, and the remainder of the skin is covered in feathers, of which there are five types, each modified for a different purpose, such as flight, insulation, camouflage and sexual attraction. There are no sweat glands, but an oil gland (preen gland) is located on the back at the base of the tail. Female chickens also have a brood patch; an area of featherless skin on the underside of the abdomen, which is well supplied with blood vessels to transfer heat to the incubating eggs.
Skeleton
The skeleton of birds must be light enough to fly and yet strong enough to support the large pectoral muscles required for flight. To achieve lightness, the medullary cavities of some of the long bones are filled with extensions of air sacs. However, this leaves the cortices of the bones considerably thinner than those of mammals, which make the bones quite brittle and susceptible to fracture. The forelimbs are modified as wings, with fusion of many of the bones to provide additional strength. The sternum of birds is massive, to allow for the attachment of the muscles of flight.
Cardiovascular system
The heart is four-chambered, and larger in proportion to body weight than that of mammals. All avian red blood cells possess a nucleus.
Respiratory system
Birds do not possess a diaphragm, and the lungs are non-lobar and do not expand as in mammals. In chickens, the lungs connect with nine air sacs, some of which extend into the long bones. Birds breathe by expansion and contraction of the chest wall, acting as bellows. Air passes through the lungs into the air sacs during inspiration, then through the lungs again on expiration. Gas exchange in birds is highly efficient, taking place on both inspiration and expiration.
Digestive system
In birds there is an oropharynx, with no soft palate. In many birds, including poultry, the oesophagus has a muscular dilatation half way along its length called the crop. This acts as a food store, and softens and regulates its flow. The stomach is in two parts, the secretory proventriculus, and the muscular ventriculus or gizzard, which grinds the food. In chickens kept outdoors the gizzard will contain grit to assist in this process. There is a short colon with two large caecae.
Renal system
The products of renal excretion are thick and pasty, high in uric acid, but with low water content. The chicken does not have a bladder, so this urate paste is excreted via the ureters, which open directly into the cloaca. The urate paste shows as a white cap on some faecal stools. Kidney failure will result in more dilute urate paste, which can be mistaken for diarrhoea.
Reproductive system
Female birds have only one functioning ovary and oviduct (the left), leading directly to the cloaca. The male has two intra-abdominal testes located by the kidneys. The ducta deferentia open into two papillae on the wall of the cloaca.
Domestic Chicken
The domestic chicken, Gallus gallus domesticus, is a subspecies of the red jungle fowl. It is by far the most numerous species of bird on Earth, with an estimated 50 billion worldwide. In the UK approximately 800 million chickens are killed and 100 000 tonnes of eggs are processed every year for human consumption.
Chickens possess a fleshy protuberance on the top of the head (the comb), and two fleshy protuberances under the chin (the wattles). These can vary in size and shape between breeds, but are usually larger on the male than the female. They act as thermal regulators, and the comb is also a sexual display organ in males.
Behaviour
Chickens are ground-living birds, unable to fly for more than short distances. They are highly social and form stable groups. The behaviours that are most important to them are nesting, perching and using substrate for scratching, pecking and dust-bathing. Given the opportunity, chickens spend a great deal of time performing foraging behaviour (pecking and scratching the ground), and foraging opportunities should be provided for chickens housed in the laboratory. Feather pecking, which can be seen in cage kept birds, is believed to be misdirected foraging behaviour, rather than aggression.
Housing
Chickens should always be kept on solid floors with a suitable substrate if possible. If cages with grid mesh floors must be used, then birds should be provided with a solid resting area occupying at least a third of the cage floor. Perches should always be available and nest boxes provided for laying birds over 16 weeks of age. Note that some hybrid strains of broiler chicken can grow very quickly, so good forward planning is required to ensure that adequate space is available for the duration of the experimental period.
Cleaning regimes will vary according to the type of accommodation. If kept in deep litter systems this is usually wood shavings or straw, which should be dry and friable. If drinker management is poor, or the ventilation is inadequate, the litter can become wet or consolidated. Bird droppings will then remain on top of the litter, which will in turn lead to soiling of the birds, scabby hocks and breast blisters, and will also predispose to respiratory infections.
Dust and dander from domestic poultry may present a risk to people with allergies or respiratory infections. Appropriate cleaning regimes, ventilation and protective clothing are therefore required.
Feeding
Chickens bred for prolific egg laying tend to be small, whereas those used as broilers or broiler breeders (meat-type) have rapid growth rates and a large body size and are less efficient egg layers. Nutritional requirements differ for these two kinds of chickens. Note that some commercial feeds contain a coccidiostat to prevent coccidiosis, and some coccidiostats for chickens can be toxic to turkeys.
For growth, laying chickens need a diet containing 18% protein initially, reducing to 16–17% as egg laying approaches (approximately 20 weeks). Diets are usually based on cereals, with a metabolisable energy content of approximately 2850 kcal (11 930 kJ)/kg of diet
Broiler chickens need up to 23% protein initially, reducing to 18% by weeks 6–8. The diet should have a metabolisable energy content of approximately 3200 kcal (13 400 kJ)/kg of diet2.
Poultry are sensitive to abrupt diet changes, so changes in feed must be made gradually by mixing the feed in decreasing proportions of ‘old’ feed over a few days. In some birds the feed intake may be restricted to control growth rate, as too rapid growth in broilers can cause lameness, or circulatory problems. It is important to feed the correct rations, and with restricted diets particularly there must be adequate space for all the birds to feed at the same time.
Newly hatched chicks need to be encouraged to feed by placing feed on chick paper, or on flat trays, where they can walk and feed easily. Chicks can survive for the first few days on the remnants of the yolk sac, but if they are not feeding by day 4 they will die of starvation (known colloquially as ‘starve out’).
Feeders must be cleaned out regularly, or food will go mouldy, risking the production of mycotoxins.
Water
This is usually provided by automatic drinkers. One nipple or cup drinker should be provided for every four birds, with a minimum of two in every enclosure. They must be cleaned regularly to prevent the build-up of pathogens. As the birds gain weight the drinkers must be raised such that the water is available at head height. Leaking and overflowing drinkers must be avoided.
Environment
The light intensity and the total length of photoperiod controls egg laying. Under natural lighting chickens often lay throughout the winter in their first season, but will go out of lay in the autumn in subsequent years, starting again as the days get longer. Increasing light intensity and photoperiod can prolong the egg-laying period. Feather pecking can be a problem in group-housed chickens, and subdued lighting has been used as a control measure to reduce the incidence of this. However, this should be discouraged, and a lighting level of at least 20 lux is recommended3. Feather pecking is better controlled by providing adequate environmental enrichment. Any increased activity resulting from the use of higher-intensity lighting can be offset by using red filters, which the birds see as dark. Wherever possible there should also be a dawn and dusk period.
Chickens should be kept at temperatures between 15 and 24°C, depending on age3. Additional heat lamps may need to be provided for young birds, in which case the birds must always have sufficient space to regulate their own environmental temperature by moving away from the lamp.
It is very important to provide efficient ventilation without draughts. Inadequate ventilation results in a build-up of airborne dust, microorganisms, water vapour and ammonia. The specialised respiratory system of birds makes them particularly vulnerable to inhalation of particulate matter and the development of respiratory infections. The presence of a significant odour on entering poultry rooms is a good indication that the air-handling system is not functioning correctly.
Birds are more tolerant than mammals to changes in relative humidity and a range of 40–80% is generally acceptable. However, extreme variation in humidity can affect the rate of heat loss in birds, which will influence feed intake and activity.
Birds are susceptible to stress and sudden or prolonged noise can have a detrimental effect on their welfare and production. The general background level of noise in a room should be less than 50 dB, below a noise-rating curve of 45, and free from distinct tonal content.
Breeding
Female chickens begin egg laying from about 18 weeks of age, and males begin to produce semen from as early as 8 weeks. Variation of the photoperiod and temperature are used to stimulate egg production in both laying and breeding birds. Most chickens bred for commercial egg production will lay an egg nearly every day from about 18 weeks of age, depending on satisfactory growth and light levels.
Under natural conditions hens lay until a certain clutch size is reached (usually 12), and then stop laying to incubate all the eggs. She will sit on the nest, and defend it if disturbed, and may not leave the nest to eat, drink or dust-bathe. The hen maintains the nest at a constant temperature and humidity, and turns the eggs regularly.
After mating, semen can take several days to reach the infundibulum, where fertilisation takes place. The fertilised oocyte enters the oviduct, where the layers of albumen are added first, followed by the two shell membranes. The main part of the shell, composed principally of calcium carbonate, is added in the shell gland at the end of the oviduct, over a period of about 20 h. The whole process from fertilisation to laying the egg takes 24 h.
The chicken embryo then requires 21 days incubation before it is sufficiently developed to hatch (28 days in turkeys). However, fertilised eggs will not begin development until the temperature rises above 21°C, and if they are stored at 12–15°C and 75% humidity they may be kept fertile for up to 14 days. The temperature can then be raised to 37.5°C by putting them in an incubator, which is known as ‘setting’ the eggs. Best results are obtained if eggs are incubated within 4–7 days of laying, and fertility drops sharply if they are stored beyond 14 days. During incubation the eggs must be kept warm and occasionally rotated. Incubators are closely controlled for temperature, humidity and ventilation, and the egg trays also gently move periodically, to mimic the action of the hen. Temperature regulation is critical, and variation from the optimum of 37.5°C reduces hatch rates. Relative humidity is also important, because this controls evaporation. Evaporation causes contraction of the fluids in the egg, resulting in the formation of an air sac between the two shell membranes. The size of the air sac, and thus the degree of evaporation, can be assessed by candling. This involves shining a bright light through the egg shell, and can be used to assess the viability of the developing embryo and the network of blood vessels surrounding it.
For best results eggs should be placed with the pointed end down and turned regularly (at least three times per day) until 1–3 days before hatching. If the eggs are not turned the embryo may stick to the shell, resulting in physical defects in the chick. Adequate ventilation provides the embryo with oxygen and prevents build-up of carbon dioxide.
Handling
Domestic chickens are normally docile and do not usually present a threat to handlers. However, they can peck, scratch or hit out with their wings, so caution is required. They are more used to human contact than most species of bird, but can become distressed if they are not handled competently. Rough handling may result in bruising or broken bones, whereas holding birds too tightly may restrict the movement of the chest wall, preventing respiration and causing asphyxia.
Chicks and young birds up to 7 weeks of age should be approached quietly and a hand placed over the bird’s back to gently restrain the wings against the body. Once held in this fashion they can be turned over, so that the chick is restrained on its back.
Adult chickens are most easily caught by placing a hand on the back and restraining the wings. Lift the bird by supporting under the body and restrain the bird by holding it against the chest. Once restrained, the hands can then be transferred to take hold of both of the legs, to support the bird’s weight (Figure 14.2).
FIGURE 14.1 Handling a chicken. (a) Pick the chicken up with both hands, ensuring the wings are restrained. (b) Hold the chicken against the body, restraining the wings and legs.
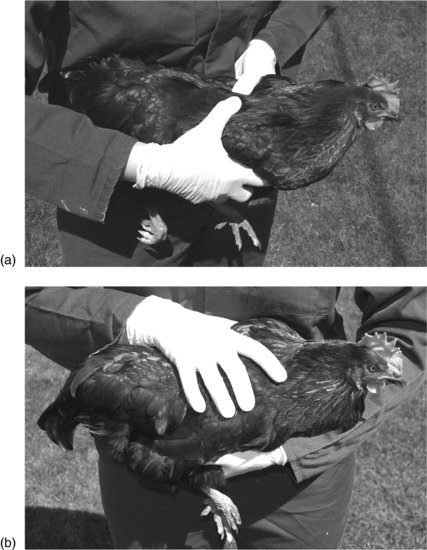
Warning: do not catch any bird by the legs, as this will lead to flapping of the wings with possible damage to the bird or the handler.
Techniques
Identification
Poultry are best identified by dyeing the feathers, or by the use of open or closed leg rings. Wing tags and electronic tags can be used, although they are more invasive. When leg or wing tags are used on chicks care must be taken to allow for growth.
Administration of substances
Intravenous
Intravenous injections are often carried out via the alar (wing) vein, found on the underside of the wing, running across the surface of the humeral–radio–ulnar joint, directly beneath the skin. Alternatively, the jugular veins run either side of the neck. Featherless tracks of skin overlie the veins and can help identification of their position.
The medial metatarsal vein is situated on the medial side of the lower leg. Care should be used with this site, as it may cause lameness.
Intramuscular
This should be performed into the pectoral muscles, which are situated on either side of the sternum. Birds have a renal portal circulation, which means that substances injected into the hind quarters of a chicken may be transported by the femoral vein to the kidneys and excreted, before entering the general circulation. For this reason it not advisable to use the leg muscles for injections.
Subcutaneous
Subcutaneous injections should be given under the skin over the keel bone.
Oral
Oral dosing is performed using a needle with a pear-drop end.
Inoculation of chicken eggs
The injection site must first be determined by candling and a small hole drilled through the shell using a glass cutter. A fine needle (13 mm, 27 G) is then passed through this hole to the required site of administration. A needle guard is used to prevent too great a penetration. The hole in the shell is sealed with melted paraffin, and the eggs are returned to the incubator.
Collection of blood
Blood is normally collected from the alar vein, or from the jugular vein if larger quantities are required. Blood removal must be carried out carefully to minimise haematoma formation. The blood may be collected directly into a micro-hematocrit tube.
Cardiac puncture must only be used for removal of blood as a terminal procedure under general anaesthesia and be followed by an approved method of euthanasia. Place the bird on its back and insert a 20–22 G needle from the neck along the ventral floor of the thoracic inlet, avoiding the crop.
Pain and stress recognition
Responses to pain in birds are more subtle than with most other species. They often adopt a hunched posture, and display wing droop. The feathers become ‘ruffled’, which may be exacerbated by lack of grooming, and the bird will separate itself from the cage mates. They may develop an increased respiratory rate and ‘mouth breathe’ when distressed. There may also be changes to the colour of the wattles or comb and evidence of oculo-nasal discharges, and the droppings may become looser.
With severe pain, birds can develop a catatonic response, where they become immobile and totally unresponsive. With chronic pain there will be a loss of appetite and weight, which can best be measured by weighing the bird on a daily basis.
Common diseases and health monitoring
Zoonoses
Domestic poultry suffer from a number of zoonotic diseases. Campylobacter and Salmonella do not usually cause clinical disease in poultry, but may cause disease in humans. Ornithosis (psittacosis), caused by Chlamydophilia psittaci, is a serious, potentially fatal, respiratory disease of humans that can be carried by many types of birds, including domestic poultry. Avian influenza is primarily a disease of birds, but there have been a number of human cases, mostly in south-east Asia, in people who have been closely associated with infected birds.
Clinical diseases of birds
There are many bacterial, viral, parasitic and metabolic diseases of domestic chickens.
Newcastle disease (fowl pest) and avian influenza can both cause rapid, unexplained, high mortality in flocks. These are notifiable and suspicion of these diseases must be reported immediately to DEFRA.
Many of the most serious chicken diseases are caused by respiratory pathogens. These include mycoplasmosis, infectious bronchitis (coronavirus) and infectious laryngotracheitis (herpesvirus). There are vaccines available for these diseases. Mareks disease and avian leucosis are also important viral infections in chickens.
Coccidiosis is extremely common in chickens. Infection with any one of nine species of this protozoan can result in weight loss, diarrhoea and death. Other parasites that infect poultry include nematode worms, such as intestinal, caecal, gizzard and tracheal worms, and ectoparasites, such as red mites and scaly leg mites.
There are a number of vaccines available for use in chickens and turkeys and these are widely used in commercial poultry farms. It is wise to check with the supplier to ascertain which diseases purchased chicks may have been vaccinated against.
More information on poultry diseases can be found at www.thepoultrysite.com.
Biological data and useful reference data
General husbandry data for chickens are given in Table 14.1. Values for feed intake and water consumption will vary with the type of bird kept and the accommodation of the birds.
Table 14.1 Biological data: chickens.
| Biological data | |
| Adult weight (kg) | 1.5–4 |
| Diploid number* | 78 |
| Food intake (g/day) | 125–250 |
| Water intake (ml/day) | 200–300 (should provide ad libitum) |
| Natural lifespan (years) | 5–8 (can live up to 30 years) |
| Rectal temperature (°C) | 41.5 (41–43) |
| Heart rate/min | 180–250 |
| Blood pressure, systole (mmHg) | 71–95 |
| Blood volume (ml/kg) | 60–90 |
| Respiratory rate/min | 15–25 |
| Breeding data | |
| Sexual maturity (weeks) | 18–24 female, from 8 weeks in male |
| Age to breed (weeks) | 20–23 |
| Incubation (days) | 20–22 |
| Clutch size | 1–14, if eggs removed birds lay daily for up to 1 year |
| Weight at hatching (g) | 50–70 g |
| Haematological data | |
| Red blood cells (×106/mm3) | 2.5–3.5 |
| PCV (%) | 22–35 |
| Hb (g/dl) | 7–13 |
| White blood cells (×103/mm3) | 12–30 |
| Hb, haemoglobin; PCV, packed cell volume. *Birds have a number of macrochromosomes that are of normal size, and also microchromosomes which are of reduced size and are difficult to count. This figure includes 60 microchromosomes. | |
Anaesthesia of chickens
All avian species present special difficulties with regard to general anaesthesia. Birds have higher metabolic rates and higher resting temperatures than mammals of equivalent size. The higher temperatures lead to greater heat loss during anaesthesia. The high ratio of surface area to body weight also increases the heat loss, so it is particularly important to minimise this during anaesthesia by maintaining a high environmental temperature and providing insulation. Avoid removing large numbers of feathers and chilling the body by application of fluids, such as surgical spirit.
Fasting pre-anaesthesia should generally be avoided as the higher metabolic rate of birds can induce hypoglycaemia. This is particularly important for birds under 1 kg body weight.
Induction of anaesthesia may be by injection or inhalation. Chicks may be induced by placing them in an induction chamber. Once anaesthetised they may then be maintained via a face mask or an endotracheal tube.
The two-stage respiratory cycle of birds and the presence of air sacs can allow a build-up of anaesthetic gases in the dependent areas of the respiratory system, which may lead to anaesthetic overload. Care must be taken not to obstruct respiration, as even short periods of apnoea can be fatal. Avoid taping the wings and legs in full extension, as this can inhibit both respiratory movements and venous return.
Birds should recover in a quiet dark environment at a temperature maintained at 40°C. All cage furniture must be removed to avoid injury. It is also a good idea to temporarily fix the wings by taping them to the body with micropore tape, to prevent damage from flapping during the recovery period.
Warm isotonic saline may be given by subcutaneous or intravenous administration, at 5 ml/kg. Birds should be encouraged to eat as rapidly as possible after recovery. Unfortunately there is little known about the effectiveness of analgesia in birds. Dose rates for anaesthesia in chickens are given in Table 9.3d.
Ruminants
Ruminants are kept worldwide for their meat, milk, skins and wool (sheep), or hair (goats). The ruminant most commonly used for scientific studies in the UK is the sheep, but small numbers of cattle, goats and deer are also used. Figure 14.3 shows a simple classification for ruminant species. Note that species in the family Camelidae, in the suborder Tylopoda, are not true ruminants. This includes camels and the South American llamas, alpacas, guanacos and vicunas.
Figure 14.3a Classification of ruminants.

Ruminants are generally acquired from commercial suppliers, which are normally commercial farms. It is not recommended that ruminants are purchased through markets or dealers, as these animals will usually have been derived from a number of different farms, with no medical or husbandry history.
Most commercially farmed ruminants are reared under conventional conditions and the husbandry and health of such animals can be extremely variable. Whenever practicable, veterinary inspection and health screening of the animals should be undertaken on the farm of origin before transport to the research facility. It is advisable to isolate all animals on arrival for a period of 4 weeks, during which time clinical assessment, tests and treatments may be carried out. The following general rules should be observed whenever possible:
- purchase from as few sources as possible,
- purchase as infrequently as possible,
- ensure the source herd is free of pathogens that are significant for the species and the research,
- isolate incoming stock for as long as possible (4 weeks, if possible),
- during isolation, medicate for endemic pathogens, such as ecto- and endoparasites.
To maintain the health status of an established farm-animal facility it is essential to impose a continuing high level of biosecurity. Personnel and animals, and feed, bedding and other equipment, are potential sources of disease. The following procedures are advised.
- Visitors should be kept to the absolute minimum and should not enter within 48 h of same-species contact. As a minimum, a full change of protective clothing should be provided before entering the unit.
- Loading and unloading procedures must be strict. All livestock vehicles present a potential disease risk.
- The proximity of other animal units and major roads should be considered when siting a new unit. Some airborne pathogens can be carried for several kilometres.
Sheep
The sheep (Ovis aries) was derived from the mouflon (Ovis orientalis) by domestication over 10 000 years ago. There are more than 200 sheep breeds worldwide, with over 60 of these found in the UK. They vary in size from small native breeds such as the Soay or Shetland (adult female 25–30 kg), to the much larger down breeds, such as the Suffolk and Oxford (adult female 85–90 kg). Breeds also differ widely in temperament and fleece characteristics, and may be either horned or naturally polled. There are approximately 14 million sheep in the UK, the majority of which are bred for meat (lamb) production. Most of the breeding females are actually F1 hybrids of two pure breeds. These ewes are then crossed with a terminal sire to produce an F2 lamb for slaughter. Unlike pigs or poultry there are no UK commercial sheep-breeding companies.
In research, sheep are commonly used for antibody production, since they are easily handled and large quantities of serum (up to 500 ml) can be regularly harvested. They are also used for studies on fetal development, reproduction, endocrinology, respiratory function and orthopaedics, as well as a number of sheep diseases of economic and public health importance.
Behaviour
Sheep are very social animals that are normally docile and tractable. At pasture they spend a large part of the day grazing over wide areas, but they are timid and have very strong flocking instincts. If disturbed they rapidly flock together, which makes them relatively easy to drive. However, their well-developed flight reflex can easily turn into a headlong rush, in which injuries can occur. They will more readily move uphill, and away from dark towards light, and this should be borne in mind when designing handling facilities. Sheep become distressed if separated from others, and should never be housed singly, unless justified for experimental reasons, in which case they must always be within sight and sound of conspecifics. Entire males can be aggressive to humans.
Housing
Sheep are able to adapt to a number of different types of housing. They may be kept outdoors at pasture, or in covered or semi-covered yards, or in totally enclosed accommodation, which may or may not be environmentally controlled.
Their thick lanolin-impregnated fleeces make them well adapted to severe weather conditions and they can remain outdoors throughout the year in the UK, providing shelter from wind and driving rain is available. Fencing needs to be close-meshed and robust, approximately 1 m high, and free of sharp projections that may cause injury. Sheep do not usually jump over fences, but will test their strength by rubbing and pushing against them until they break. Outdoor housing has many environmental advantages for sheep, but they will be exposed to both internal and external parasites, and parasite-control programmes will be need to be drawn up. Young lambs may also be liable to predator attack (from dogs or foxes).
If sheep are kept indoors it is advised that they are kept in groups of no more than 30, matched by age and size to prevent bullying at feeding. Horned sheep should not be housed in the same enclosure as polled sheep.
The walls and doors of the enclosure should be non-porous and easy to clean. The lying area must have a solid floor, made of concrete or rammed chalk, which gives better drainage. Bedding should preferably be deep straw to provide insulation and comfort, although wood shavings or sawdust may be alternatives. Fresh, clean, dry bedding should be added on a regular basis, until the height of the bed requires its removal. The pen should then be washed down and allowed to dry before re-bedding.
Environmental enrichment can be difficult with sheep. Toys, such as balls or chains, do not seem to be of much interest to sheep. The provision of conspecifics, roughage and space to lie down to ruminate is probably the most important enrichment for housed sheep. Un-chopped root vegetables, such as sugar beet, are well liked and give them something to gnaw at. They may be provided as a treat on a daily basis, although in the long term there is a risk of damage to the incisor teeth.
Breeding ewes are usually housed in individual pens with their lambs for a few days at lambing time. This ensures that the lambs receive colostrum and develop the maternal bond. After 24–48 h ewes and lambs can be mixed with others in small groups.
Feeding
Sheep are obligate herbivores and their digestive tracts have adapted to a diet of vegetable matter. They have no upper incisor teeth, but in their place possess a hard, ridged fibrous ‘dental pad’, against which the lower incisors bite. They have no canine teeth, leaving a gap, the diastema, between the lower incisors and the molar arcades. The molars are continuously growing and adapted for grinding fibrous food. The jaw is articulated to allow movement from side to side for efficient chewing.
The non-glandular fore-stomach consists of three large chambers: the reticulum, the rumen and the omasum. The rumen and reticulum contain bacteria that digest cellulose, releasing fatty acids, and produce vitamins. Food from the rumen is regurgitated several times for extra chewing, in a process known as rumination. A side effect of the breakdown of cellulose is the production of large quantities of methane gas. This is removed from the rumen by frequent belching (eructation), which may occur as often as once every minute. If ruminants cannot release the gas produced, the methane will accumulate to distend the rumen, causing the condition commonly known as ‘tympany’ or ‘bloat’. In severe cases the pressure of the distended rumen will cause circulatory disturbance and, unless relieved, death.
Food eventually passes from the rumen, through the omasum, which absorbs much of the fluid, to enter the abomasum, which is analogous to the glandular stomach of other mammals. In young lambs the rumen is undeveloped and milk passes via a muscular groove (the oesophageal groove) to enter the abomasum directly. The rumen gradually develops in response to the ingestion of roughage, and is fully functional by about 8 weeks of age. Sheep produce copious quantities of saliva, but do not vomit. However, drooling and spillage of regurgitated rumen contents may be seen.
Sheep are grazing animals and will nibble grass and vegetation at ground level. Under most circumstances they will obtain sufficient nutrients for growth and maintenance from grass alone. The optimum sward height for sheep is 2.5 cm, and they can have difficulty utilising very long grass. If the grass quality or availability declines sheep should be fed a long-fibre conserved forage, usually hay but alternatively silage or even straw. In some circumstances, such as pregnancy and lactation, the ewe’s diet may require supplementation with higher energy ‘concentrate’ foods. Concentrates are normally based on cereals, with additional protein, vitamins and minerals. Approximately 125 g/head per day of a commercial sheep food should be fed daily from day 100 of pregnancy, gradually increasing to as much as 1 kg/head per day at parturition. Sheep fed on straw as their sole source of roughage overwinter, will require additional concentrate feeding.
Whenever sheep are kept indoors roughage should be permanently available in hayracks. If concentrate rations are also fed there must be sufficient room at the feed trough for all animals in the pen to feed at the same time. Where horned animals are kept additional space allowance must be made at the feed troughs to avoid injuries.
Mineral deficiencies may occur if the pasture or conserved foods are deficient in copper, cobalt or selenium. Supplementary minerals may be advised in these circumstances, but great care is required in their formulation, as sheep are peculiarly susceptible to copper toxicity. Concentrate rations or minerals intended for cattle or pigs contain added copper and should never be given to sheep, as they can induce copper toxicity.
Poor dentition can be a problem in older sheep. The incisor teeth are frequently lost. However, the majority of sheep can maintain satisfactory body condition without incisor teeth. Of more importance are the molar teeth, which are essential for masticating food. Loose or infected molar teeth are a common cause of weight loss in older sheep.
Growing sheep are particularly prone to urinary calculi when fed high levels of concentrate. In male lambs (entire or castrated) the urethra is narrow, and calculi may cause urethral blockage, with urine retention and fatal consequences. To prevent this it is essential that the magnesium and phosphorus content of concentrate rations be restricted. Salt licks should always be provided to encourage drinking and urinary throughput, and urinary acidifiers may also be helpful.
Water
Sheep must be provided with clean water ad libitum at all times. They prefer to drink from flowing water, although this may be difficult to achieve in practice. Static water tanks are acceptable, but care is required because sheep can become cast in them and drown. Bowl drinkers are satisfactory in indoor pens, but nipple drinkers are not suitable for sheep.
Environment
Sheep will tolerate a very wide range of temperatures. The thermoregulatory neutral zone for sheep is 10–30°C, but the extremes of this range depend on whether they have a full fleece or not. Unshorn sheep in summer can suffer from heat stress, exhibiting panting and mouth breathing at temperatures over 20°C. This is exacerbated if they are kept indoors, and in these circumstances shearing should be undertaken in April or May, well in advance of any hot weather. Once shorn, sheep are more susceptible to cold and should not be exposed to temperatures of less than 10°C. If shearing is undertaken very early in the year they must be housed for at least 2 months before they are turned outside, to allow some fleece re-growth. Sheep permanently housed in environmentally controlled conditions will generally benefit from shearing twice a year, and should be kept at between 10 and 24°C. Adequate ventilation is essential to reduce the risk of respiratory disease. Extractor fans should remove 3 m3 air/kg of body weight per hour to control ammonia levels and humidity levels should be kept between 45 and 65%.
Breeding
Sheep are seasonally polyoestrous, returning into oestrous every 16–17 days over the winter period (September–February). The onset of the breeding season is controlled by diminishing day length, but varies significantly between breeds. Normally the hill breeds start later (October–November) than the lowland breeds (August–September). Dorset sheep are unusual in having a very long breeding season, extending from about June to March. This means that they may be mated every 8 months, giving birth to three litters in 2 years. Dorset breeds and their crosses, particularly the prolific Finn-Dorset, are popular research animals.
Under farm conditions ewes are normally mated in the autumn, to give birth the following spring. They run with the rams (tups) for a period of about 6 weeks. Ultrasound scanning at 50–90 days of gestation is widely practised to determine pregnancy and count fetal numbers. Ewes carrying multiple fetuses can then be grouped separately for differential feeding.
Gestation lasts approximately 147 days (range 142–150 days), although there are slight breed differences. Ewes carrying multiples normally have shorter gestation times than those carrying single lambs, but as a rough guide, in the northern hemisphere, ewes mated on Guy Fawkes day (5 November) will lamb the following All Fools Day (1 April). Ewes may be mated as lambs (ewe-lambs) in their first autumn, at about 6 months of age, provided their body weight is at least 70% of the adult weight for the breed. Alternatively, they may be left until the second autumn, when they are 18 months of age. Weaning normally takes place at 12–16 weeks, although there are management systems where weaning may be as early as 6 weeks, provided suitable concentrate rations are provided.
In most sheep breeds the breeding season can be advanced by a number of methods. These include the use of progesterone-impregnated intra-vaginal sponges, melatonin implants or the use of vasectomised males. By advancing the breeding season in the UK to July or August lambs can be produced in December/January. Artificial insemination (AI) is possible in sheep using either fresh or frozen semen, and embryo transfer may also be carried out by laparoscopy under general or local anaesthesia.
Handling
Sheep are particularly easily startled and patience and gentle handling are required for success. They are inclined to follow one another, and will generally move away from humans, dogs or buildings towards open countryside. Where larger numbers are involved a proper sheep-handling system should be employed.
Where sheep are held indoors carefully designed housing, consisting of holding pens leading through narrow walkways and races to smaller pens, will allow individuals to be caught with minimal stress. Where such facilities do not exist the best method of restraint is to gradually pen the sheep into a corner using a number of hurdles. A trained handler can then restrain the individual sheep, by holding one arm under the neck and the other around the rump (see Figure 14.4). Sheep should never be caught by the wool, as grasping the fleece is painful and may result in handfuls of wool being torn out. It is also not advised that sheep be caught by their horns. It is an offence under welfare legislation to lift a sheep by its horns.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



