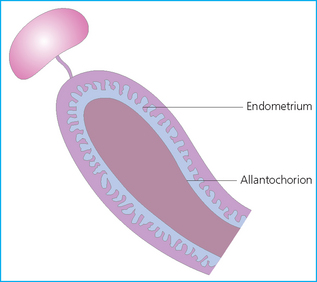
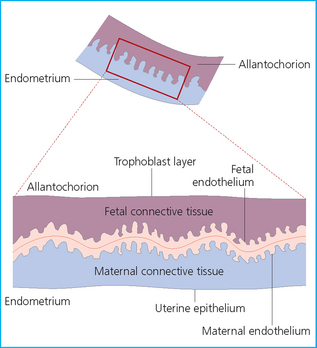
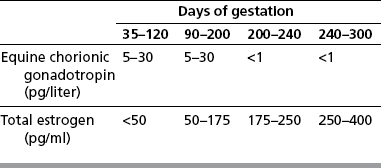
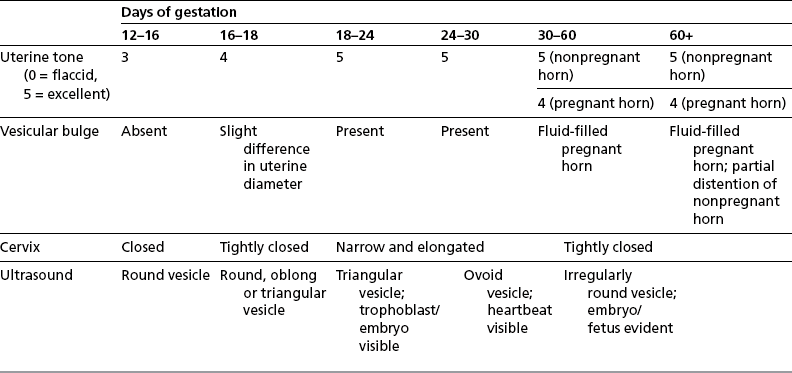
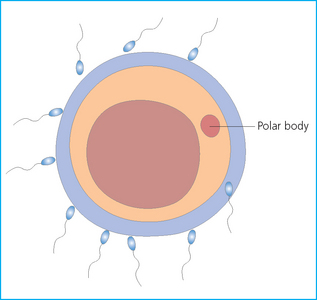
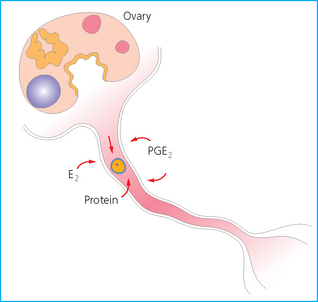
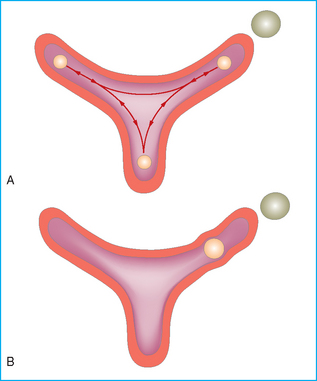
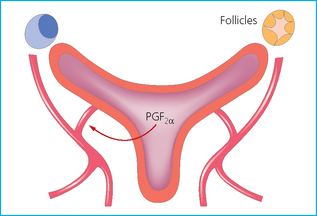
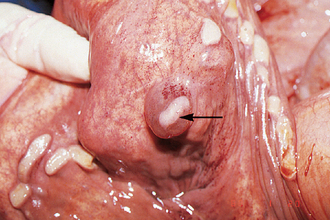
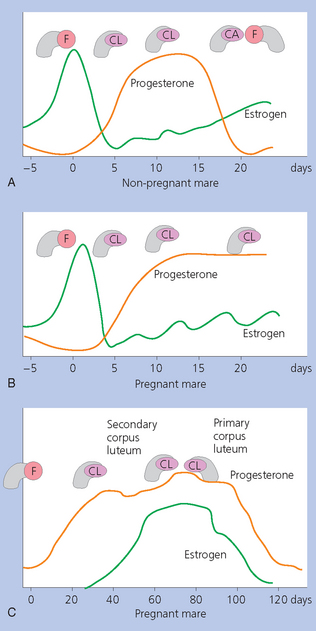
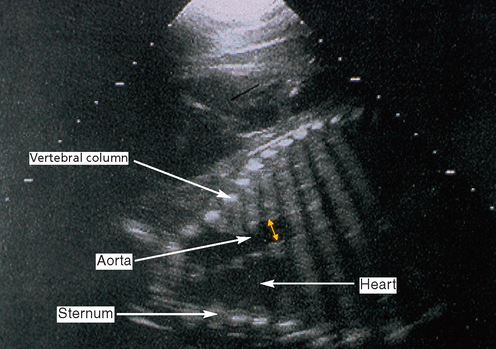
Chapter 7 The objective of a successful reproductive program is to produce the maximum possible number of live, healthy and athletic foals each year from the pool of mares at stud.1 After an uneventful delivery, the foal should grow into a productive adult. The economic consequences of a good or bad fertility for a stud are incalculable. Failure to realize the potential of a valuable stallion results in a poorer value and an economic downturn; the converse is also true. The benefits of successful progeny are easy to appreciate. In stud medicine, assessment of fertility is taken to include the realization of athletic performance of the progeny. Without a successful progeny record even the best performing stallion will inevitably end up with a lower commercial value/reputation than a stallion whose progeny consistently achieve success in the chosen performance arena. • The characteristics of a normal pregnancy. • The factors involved in a normal or successful delivery and the management of: Pregnancy occurs as a result of fertilization of an oocyte with a spermatozoon, and subsequent maturation of the ensuing embryo with its passage into the uterus (Fig. 7.1).3–5 The ovum has undergone one meiotic division close to ovulation and the first polar body is typically extruded before it enters the oviduct. Fertilization of the oocyte with a single spermatozoon occurs in the ampulla of the oviduct; this stimulates the completion of meiosis and the early development of the embryo. The embryo enters the uterus as a compact morula 5–6 days after ovulation. The uterine proteins and secretions provide a suitable environment for the developing embryo. Fig. 7.1 Fertilization of the oocyte. A single spermatazoon will penetrate the oocyte, blocking the penetration of further spermatozoa. Typically, only fertilized eggs will pass from the oviduct into the uterus. The mechanism that allows a fertilized egg to enter the uterus, but precludes an unfertilized one, is unknown.6,7 Prostaglandin E2 (PGE2), oxytocin and local hormones, or even proteins produced by the embryo and/or uterine tube themselves, may stimulate embryo transport (Fig. 7.2). Fig. 7.2 Transport of the embryo through the oviduct. This is facilitated by the production of prostaglandin (PGE2), oxytocin, local hormones (E) and proteins by the ovary, endometrium, oviduct, and embryo. Once the embryo enters the uterus, it sheds its original outer coating (the zona pellucida) and develops a new smooth capsule as a result of the action of uterine proteins or secretions.8 This capsule protects the embryo during its stage of active migration through the uterus. The capsule is not shed until the amnion is complete, at around day 20 of pregnancy, thereby providing protection to the embryo proper until its placental protection is produced. The capsule may also provide protection from maternal white blood cells and antibodies. Although a chemical factor associated with early pregnancy (early pregnancy factor) can be recognized as early as 48 hours after conception,9 the pregnancy is very unstable at this stage and so its use for pregnancy diagnosis is probably not helpful. The embryo migrates through the entire uterus from day 6 until day 15–16 post ovulation.10 Movement is maximal between days 11 and 14. The migration appears to be necessary for maternal recognition of pregnancy. The embryo contacts the entire endometrial surface repeatedly as a result of uterine contractility and continues until the size of the embryo precludes its movement, along with an increase in uterine tone due to persistently high levels of progesterone. The cessation of movement of the embryo is called fixation (Fig. 7.3) and typically occurs at the base of one of the uterine horns.11,12 The embryo is thought to produce factors that prevent release of PGF2α from the endometrium, thereby allowing maintenance of the corpus luteum within the ovary. The developing embryo will begin to produce estrogens at about day 12 of pregnancy. Estrogens may increase the mobility of the embryo. The production of estrogen from the embryo and progesterone from the primary corpus luteum results in a marked increase in tone in the uterus.13 This is one of the cardinal diagnostic signs of early pregnancy. Fig. 7.3 Embryo motility and fixation. (A) Mobility phase: in early pregnancy when uterine tone is good and the embryo is small, uterine motility moves the embryo along the entire lumen of the uterus. Embryonic factors produced by the embryo and/or endometrium are released, resulting in maternal recognition of pregnancy. The embryo will migrate throughout the entire uterus many times. (B) Fixation phase: as uterine tone increases and embryonic size increases, the embryo becomes trapped in the uterine lumen. Fixation usually occurs near the body/bifurcation junction (more rarely in the body or tip of a horn). Maternal recognition of pregnancy occurs between days 14 and 16.3–5 This is the point at which PGF2α would normally be produced to result in regression of the corpus luteum and the instigation of the next estrous cycle. In the pregnant mare this does not occur and so the next cycle is suspended. PGF2α is normally produced by the endometrium and is carried by the local circulation to the ovary with the corpus luteum, resulting in the latter’s demise (Fig. 7.4).3–5 The embryo must be present in the uterus for maternal recognition of pregnancy. It has yet to be proven if the embryo produces a factor that blocks or inhibits PGF2α production by the endometrium. Movement of the embryo along the endometrial surface until days 15–16, when it becomes fixed within one of the horns, blocks the release of PGF2α from the endometrium. Blockage of PGF2α release maintains the corpus luteum. The embryo must have access to at least 50% of the uterine lumen for maternal recognition of pregnancy to occur. Fig. 7.4 Luteolysis. PGF2α is produced by the endometrium, released into the local circulation and carried to the ovary, resulting in the demise of the corpus luteum. • The equine conceptus is called an embryo until day 40 of pregnancy and is called a fetus thereafter.21 At or immediately before delivery it is commonly referred to as the foal. • Attachment of the placenta to the endometrium occurs gradually, beginning around days 36–40 of pregnancy, and continues until day 150 when placentation is complete. • Around day 25 of pregnancy, endometrial cup formation begins.22 The endometrial cup cells are specialized trophoblasts produced by the developing conceptus that form the chorionic girdle, an annular band of tissue which invades the epithelium of the uterus starting at about 35–38 days of pregnancy. There is a generous supply of lymphatic vessels in the area of the endometrial cups; this is probably important for the transport of lymphatic fluid involved in the immune recognition of the embryo by the maternal immune system. The endometrial cups can be seen on the endometrial surface as pale shallow cups of tissue in the pregnant horn. The endometrial cups produce equine chorionic gonadotropin (eCG; formerly known as pregnant mare serum gonadotropin). The cups continue to increase in size until day 70 of pregnancy at which time they begin to degenerate (Fig. 7.5). They will continue to produce eCG until days 120–150 when degeneration is complete; they then cease functioning completely. eCG is also thought to assist in immune regulation during early pregnancy. Fig. 7.5 Endometrial cups (day 70). Note the normal interrupted ring of cups on the uterine wall. The arrow indicates an endometrial cup implanted on a uterine cyst. • During the first 3 weeks of pregnancy the yolk sac is the predominant structure of the newly forming placental unit. At around day 30 of pregnancy, the formation of the allantois begins to dominate the placental unit. The yolk sac is visible through the second month of pregnancy. The allantois and the chorion will fuse and attach to the endometrium to form the placenta, which will be present for the remainder of the pregnancy. The formation of the placenta occurs slowly. The entire endometrial surface is encompassed with placental attachment by day 80 of pregnancy. The allantochorion will invade the endometrium by the attachment of tiny finger-like structures known as microvilli or microcotyledons (Fig. 7.6).23 These tiny invasions of the endometrium allow fetal nutrition and facilitate waste management and transference of substances (proteins, hormones, etc.) across the placental membranes. Microvilli are initially noted by day 40 of pregnancy and continue to develop beyond day 100. They provide for the exchange of nutrients, blood, oxygen, and other molecules between the mare and the fetus (Fig. 7.6). Placentation in the mare is described as epitheliochorial.24,25 This term is used to describe the fact that the maternal endometrial epithelium and the fetal chorion are intact and this has implications for the function of the placenta. There are six layers involved in the placenta of the mare (Fig. 7.7): • The maternal vascular endothelium (lining of the blood vessels on the maternal side). • The maternal connective tissue. • The fetal connective tissue. • The fetal vascular endothelium (lining of the blood vessels on the fetal side). The hormonal profile during the first 2 weeks of pregnancy is similar to that of the non-pregnant mare (Fig. 7.8). The differences in hormone concentrations start at day 14, when prostaglandin would normally increase with a subsequent decrease in progesterone. It is the maternal recognition of pregnancy that accounts for the continued production of progesterone because prostaglandin is not released. Maternal recognition of pregnancy has been described on p. 228. The primary corpus luteum (corpus luteum of ovulation) is the sole source of progesterone until day 35. Fig. 7.8 Hormonal profile of (A) the nonpregnant mare and (B) the pregnant mare during the first 20 days of pregnancy, and of (C) the pregnant mare during the first 120 days of pregnancy. F, follicle; CL, corpus luteum; CA, corpus albicans. At day 25 endometrial cup formation begins, followed by the production of eCG from the cup cells at around day 36.27,28 eCG has biologic activities similar to those of follicle-stimulating hormone and luteinizing hormone. The function of eCG is unclear. It was previously thought that it stimulated development of secondary follicles and the formation of secondary or accessory corpora lutea. However, experiments have established that continued rhythmic secretion of follicle-stimulating hormone during pregnancy stimulates growth of secondary follicles.29,30 The luteinizing hormone-like component of eCG causes luteinization and/or ovulation of already mature follicles. This luteinization is considered to be responsible for the rise in serum progesterone concentrations observed between days 40 and 70. eCG stimulates primary and secondary corpora lutea to continue producing progesterone until days 100–120 of pregnancy. By 150–200 days of gestation all corpora lutea will have regressed. The supplemental progesterone produced by the secondary corpora lutea assists in the maintenance of pregnancy until the placenta is fully functional (Fig. 7.9). eCG may be also involved in the formation of an immunoprotective barrier that slows rejection of the ‘foreign’ fetal cells during the first half of gestation31. Fig. 7.9 Multiple corpora lutea may assist the maintenance of the early pregnancy prior to the development of a fully functional placenta. The combination of fetus and placenta makes significant contributions to the endocrine patterns of pregnant mares. The unit is responsible for the production of progesterone, progestins, estrogens, and relaxin. Before day 60, a small amount of circulating progesterone and 5α-pregnanes (progestins) are produced by the fetoplacental unit. At around day 90, serum concentrations of 5α-pregnanes increase, only to level off at about 180 days of gestation. Concentrations remain relatively constant in maternal serum until days 305–310 of gestation when they begin a dramatic rise, peaking about 5 days before parturition.33 The rise in progestins in the last 3 weeks of gestation is thought to be associated with maturation of the fetal adrenals. Uteroplacental progestin production rises in late gestation, concurrent with an increase in fetal pregnenolone output probably from the fetal adrenals which increase in weight during the last month of gestation. The decline in plasma progestins over the last 1–2 days prepartum occurs in parallel with a substantial surge in fetal plasma cortisol and maturation of the equine fetus.34 The fetoplacental unit also produces significant levels of estrogens, some of which are derived from the fetal gonads and some from the placenta.35 There are eight types of estrogenic compounds produced by the fetoplacental unit. A second surge in estrogens occurs around day 60 of pregnancy when estrogens synthesized by the fetoplacental unit appear in the circulation. A massive rise continuing from day 70 to day 100 is only likely to occur in mares with normal fetal development. Estrogen production appears to follow gonadal size closely, peaking at about day 210–240, and gradually decreasing, though still detectable, as gestation continues to term. The elevated levels of conjugated estrogens can be used as a pregnancy test in the horse by immunoassay of blood, urine or milk from day 45 to term (Fig. 7.10). Fig. 7.10 Estrogen elevation in blood, urine or milk can be used as a test of a viable pregnancy after day 45. eCG, equine chorionic gonadotropin. • Measuring levels of total estrogens in the blood or urine of the pregnant mare can assess fetal viability. • Depending on the stage of pregnancy, either total estrogens or eCG can be measured to determine the normality of fetal development (Table 7.1). • eCG is also believed to be involved in stimulating estrogen production from the ovaries in early pregnancy (beginning at day 35 of pregnancy). eCG is thought to stimulate estrogen production through its ability to invoke luteal steroidogenesis. • eCG is secreted by the endometrial cups. It is detectable as early as day 35, peaks around day 60 and then gradually falls, to disappear by day 125. • eCG has follicle-stimulating hormone and luteinizing hormone properties. • eCG drives the formation of secondary corpora lutea (through its luteinizing effects)36 from the large number of follicles that form from the action of pituitary follicle-stimulating hormone. • The luteinizing effects result in the rise in serum progesterone concentrations between day 40 and day 70 and may also be responsible for some of the antirejection effects that enable the embryo to survive rejection by the uterine environment. • Measurement of eCG is a useful indicator of pregnancy between day 40 and day 120. • The estrous cycle is blocked by eCG production from the endometrial cups. This explains why, once a mare is pregnant beyond 40 days, she will not return to estrus until the cups regress at around day 120 from the ovulation date. Once formed, the endometrial cups persist up to day 120 whether pregnancy is maintained or not. Therefore, false-positive pregnancy tests (for eCG) are possible if the mare has lost the pregnancy after day 40. • During the period when the endometrial cups are dominant, neither prostaglandin nor saline infusions will be effective in inducing a fertile estrous cycle. • The fetoplacental unit (comprising the fetus itself and the placenta) produces progestins. • In the early stages of pregnancy (before day 60) the fetoplacental unit produces a small amount of progesterone. • Small amounts of progesterone can be detected in the mare’s blood before 5 months and after 10 months.37 However, total progestins remain high for the duration of the pregnancy; this is due to the secretion of other progestins (such as 5α-pregnane) by the fetoplacental unit. • Serum progesterone assay (in which total progestins are assayed) may therefore be used to indicate pregnancy after day 60. • The rise in total progestins in the last month of pregnancy corresponds with maturation of the fetal adrenal glands. Increases in fetal adrenocorticotropin in late gestation stimulate the fetal adrenal glands to produce pregnenolone. The fetal adrenal glands cannot convert progesterone to cortisol as it is lacking the enzyme 17α-hydroxylase needed for conversion. Therefore, pregnenolone is converted to progesterone which is likely reduced to progestins within the fetoplacental unit.38 • A premature rise in progestins (before 305 days) indicates fetal stress or precocious maturation, whereas a drop is usually observed in fetal demise.34,39 • From day 35, the concentration of estrone sulfate in the mare’s blood increases. This is also reflected in a urinary increase. • Estrone sulfate is predominately secreted by the developing fetus and the fetal membranes.40 • Estrone sulfate is an index of fetal viability as well as of pregnancy. Because this hormone is produced only by a viable fetus, any drop in urinary estrone sulfate concentration (and less sensitively in the mare’s blood) between day 60 and term should be viewed as indicating fetal death/compromise. Almost no foal is viable when this occurs. Safe, early and reliable diagnosis of pregnancy is an essential part of stud farm management, without which overall fertility rates would probably be much lower. There are obvious advantages to establishing that a mare is pregnant and that the pregnancy is (at least at the stage of the examination) normal. Early embryonic death is a relatively common event in mares (see p. 240) and the development of a pseudo-pregnancy may seriously affect the future fertility of the mare. A standard protocol of diagnosis therefore needs to be used to define the best stages at which to confirm a pregnancy. Early loss of a pregnancy or a nonpregnancy-related cessation of cyclicity might result in serious financial loss for the owner. However, at least if the problems can be defined early, suitable measures may be taken to re-establish a pregnancy. Furthermore, one of the most important aspects of diagnosis is the detection of twin pregnancy at a stage at which manipulations are possible (or at least so that reproductive cyclicity can be restarted completely). Pregnancy can be diagnosed by a variety of methods. A diagnosis of pregnancy may be suspected by nonreturn to estrus at 17–21 days post ovulation. In a breeding mare, the absence of estrus must be regarded as strongly suggestive of pregnancy. However, not all mares that fail to demonstrate estrus will be pregnant because of a variety of factors, including, but not limited to, early embryonic death or retained corpus luteum. False-positives and false-negatives are common when estrous behavior alone is monitored. • Estrous behavior may be ‘silent’ or at least not overtly visible in mares that are out of contact with stallions. • The length of the estrous cycle is sufficiently variable to make this an unreliable method. • Nonpregnant mares (particularly lactating mares with foals at foot early in the season) may not return to estrus after mating, thereby giving the impression of pregnancy. • Some mares will show prolonged diestrus due to persistence of the corpus luteum. • Pregnant mares may show estrous behavior. • Palpation of a 16–19-day pregnancy will indicate the existence of good to excellent uterine tone (the uterus feels singularly firm and turgid), and the cervix will be tightly closed (Fig. 7.11). Fig. 7.11 Rectal examination of an early pregnancy at: (A) 33 days; (B) 42 days; (C) 60 days; (D) 90 days. The obvious palpable characteristics are: characteristically excellent uterine tone; a ventral bulge in the pregnant horn; a narrow elongated cervix. • By day 20 the embryonic vesicle can be palpated by an experienced practitioner using rectal examination only. However, it is difficult to determine if twins are present at this stage of the pregnancy by palpation alone, especially if they are unilaterally fixed (see p. 242). • At day 20–21 there is a palpable ventral bulge at the uterine bifurcation, uterine tone is very good to excellent and the cervix is becoming narrow and elongated. • By day 30 the ventral enlargement of the pregnant horn is quite prominent. Uterine tone directly surrounding the bulge is decreased, but tone in the tip of the gravid horn and of the entire nongravid horn is very good to excellent. The size of the bulge continues to increase, while the tone surrounding it decreases with time. • By day 50 the uterine fluid involves the mid-uterine horn and the uterine body at the bifurcation. The uterine horn tips still have good to excellent tone. The cervix remains tightly closed throughout the pregnancy. • As the pregnancy progresses, the uterus increases in size and gravitates into the abdominal cavity. The ovaries may be noticeably closer together (more towards the midline). Because the fetus is small and there is significant fluid surrounding it, actual palpation, or ballottement, of the fetus is not usually possible until much later in the pregnancy. The uterus of a mare with a pyometra (see p. 183) may be mistaken for a pregnancy of 70 days or more, but the uterus will have a doughy ‘dull’ feel, which is quite atypical of a pregnancy. • Rectal palpation of the fetus is easily performed beyond 120 days of pregnancy, as the fetal size increases in relationship to the volume of uterine fluid. It becomes increasingly easy to identify the fetus and so it may be possible to estimate the age of a fetus by comparison with standard measurements for head or limb size (see Fig. 7.12). Direct visualization of the cervix using a vaginoscope (or an endoscope), or direct palpation, can be used to aid the diagnosis of pregnancy.42 Vaginoscopic examination has largely been replaced by rectal palpation. Nevertheless, detectable changes are obvious at an early stage. In early pregnancy the cervix has a pale appearance and is tightly closed.43 It is frequently pulled to one side in the vagina (Fig. 7.13). The value of ultrasonographic examination for the diagnosis and assessment of pregnancy is undisputed; it is probably the most important advance in our ability to monitor pregnancy.44 Its ability to detect very early pregnancy with a high degree of accuracy (with an experienced operator) means that fertility rates can be significantly improved.45 Modern ultrasound machines are small, portable and robust. The definition obtained by these is often remarkable and very subtle changes can easily be detected. Apart from its obvious benefits in pregnancy diagnosis, ultrasonography is routinely used to investigate uterine and ovarian health and status. The procedure itself has no known dangers, although the risks of rectal examination should never be ignored. • Before day 14. Examination for pregnancy before day 14 requires careful examination of the uterus with a high quality 5- or 7.5-MHz ultrasound transducer. Ultrasonography can be utilized to evaluate pregnancy between days 9 and 16 before any detectable palpable changes will be present. The ultrasonographic characteristics of the early embryonic vesicle are shown in Fig. 7.15. The embryonic vesicle is about 2 mm in diameter on days 8–9 of pregnancy. Fig. 7.15 Ultrasonographic appearance of a developing embryo at: (A) 10 days; (B) 17 days; (C) 18 days; (D) 21 days; (E) 23 days; (F) 28 days; (G) 40 days; (H) 50 days; (I) 65 days. • Days 16–19. From a practical standpoint, ultrasound examination can often be delayed until days 18–20. The embryonic vesicle is typically 15 mm at 15 days of gestation. • Day 18. The ultrasound evaluation will reveal a slightly elongated or triangular shape to the vesicle and the outline is less regular. • Days 19–20. The trophoblast will be noted first on the ventral surface of the vesicle. It will subsequently slowly migrate dorsally as the yolk sac regresses and the allantois develops beneath the embryo. • Days 20–21. The trophoblast will slowly migrate dorsally. • Days 24–50. A heartbeat is evident in the embryo proper on average around day 24. This is the first time at which fetal viability can be assessed. • Days 28–50. The trophoblast is seen near the middle of the vesicle and by day 40 it is near the dorsal edge of the vesicle. At this time the umbilicus begins to elongate and the fetus will gravitate ventrally again, reaching the bottom of the vesicle by day 50. • Days 50–180. The increasing size of the fetus makes it more difficult to image the whole structure; however, the fetal fluids and individual anatomical parts can almost always be identified. • By day 180 of pregnancy, the fetus can be examined by transabdominal ultrasound as well as transrectally (Fig. 7.16). Visualization of the heartbeat is often easier with transabdominal ultrasonography. The mare’s abdomen may need to be clipped to facilitate this examination. Fig. 7.16 Transabdominal ultrasonogram of a 6-month fetus obtained by placing the probe on the ventral abdominal wall on one side of the midline. The mare’s skin surface is at the top of the picture and the foal’s thoracic vertebrae and rib pattern are obvious (arrows). The foal is of course lying on its back at this time. Note also the fetal heart open and aorta (label), which can be clearly seen during the examination. Pregnancy diagnosis can also be made by hormonal means. These tests are indirect indicators of pregnancy and can be misleading when used on their own; they are therefore regarded as unreliable and should not be used as the sole source of pregnancy determination. Progesterone concentrations can be determined easily and quickly in blood or milk samples and the results are probably better than reliance on absence of estrus alone.46 The test kits are accurate and suitable for use in practice. Progesterone can be measured in a practice laboratory using an ELISA kit. • At 18–20 days post ovulation, pregnant mares should have a peak progesterone concentration above 2 ng/ml, while a nonpregnant mare should be returning to estrus and have a progesterone concentration below 2 ng/ml. Progesterone concentrations range from 4 to 10 ng/ml in most mares in early pregnancy. However, not all mares with these concentrations will be found to be pregnant, and some pregnant mares have lower progesterone concentrations for short periods. • Progesterone concentrations may increase considerably after day 20. A persistently elevated progesterone after days 17–21 post ovulation is suggestive of pregnancy; however, progesterone may remain elevated in: • Progesterone concentrations remain elevated until days 150–200 of pregnancy and then decline. After day 200 they may be as low as <2 ng/ml and should not be used as the sole means of pregnancy determination. • During the last month of pregnancy, progesterone levels increase slightly until 5 days prior to parturition. As described previously (see p. 228), the fetal trophoblasts that invade the maternal endometrium produce detectable levels of eCG at around days 35–38. These cells form the endometrial cups, which maintain their function of stimulating secondary ovulations until around day 60 when maternal rejection causes their remission. The cup cells begin to degenerate after about day 75 and finally cease to function at around day 120. • Early embryonic death that occurs prior to endometrial cup formation (before days 32–35) will result in a return to estrus within 21–28 days. However, if endometrial cup formation has started, and eCG production has begun, follicular development and secondary corpus luteum development will occur whether the mare remains pregnant or if the pregnancy is lost. Because the supplemental corpus luteum will have formed, the mare will not return to estrus until endometrial cup degeneration is complete, which is usually around 120–150 days from ovulation. Therefore, if eCG test kits are utilized to determine pregnancy status without rectal confirmation of pregnancy, a false-positive pregnancy test is possible. The concentration of estrogen in urine, blood or feces can be used to diagnose pregnancy after day 60 up to full term3–5 using standard ELISAs for conjugated estrogen determination. • Although a minor and insignificant maternal increase in estrogens is common between 35 and 60 days (as a result of pulsatile secretion of follicle-stimulating hormone), conjugated estrone sulfate concentrations can be used to diagnose pregnancy after day 60.51 • Estrogen concentrations increase until days 180–240 days of pregnancy reaching their peak in blood (and urine) around 150 days and then slowly decline until birth. In contrast to other indirect indicators of pregnancy, estrogen levels are good indicators of fetal viability. Death of the fetus results in an immediate decrease in circulating estrogen concentrations.2 • If a mare in late gestation begins to lactate, fetal viability may be established by measuring estrone sulfate, although ultrasonographic examination can provide much more information. As long as the approximate stage of pregnancy is known, the serum estrogen concentration of the dam can be compared to the normal range of values for that stage of pregnancy. • Although ultrasonography has revolutionized pregnancy diagnosis, it is not a replacement for rectal palpation. Ultrasonography cannot measure uterine and cervical tone, nor can it detect the presence of some abnormalities of the broad ligaments, ovaries, uterus and cervix, which are important factors in the assessment of a pregnancy. • Additionally, a solitary uterine cyst may be confused with a vesicle, resulting in the misdiagnosis of pregnancy. • Early embryonic death may be difficult to distinguish using either palpation or ultrasonography until sufficient changes occur to differentiate it from a normal pregnancy. • Estrogen concentrations in blood and urine are useful adjuncts to assess fetal viability when other means are not practicable. • Pregnancy is most accurately diagnosed using a combination of rectal palpation and ultrasonography (see Table 7.3).3–5 The benefits include the ability to: Table 7.3 Methods and accuracy of pregnancy diagnosis Determination of fetal sex is becoming more significant and can be performed with a reasonable degree of accuracy between days 55 and 75.55 In order to perform this procedure it is important that: • The operator is experienced. Experience can be gained during normal ultrasonographic examinations carried out between 59 and 65 days. • The pregnancy is between 55 and 75 days. Proper timing is essential! • High-quality ultrasound equipment is used. • Adequate restraint and rectal techniques are used (to avoid risks of injury to mare and operator and allow a relaxed examination). • There is subdued external light so that the image can be clearly seen. When undertaking the examination it should be noted that: • The genital tubercle appears as a bi-lobed structure approximately 2 mm in diameter (Fig. 7.17). Fig. 7.17 Ultrasound scan of an equine fetus at 60 days showing the genital tubercle of a female foal (arrow) (slide courtesy of J. Pycock). • The genital tubercle is located close to the umbilicus in the male fetus and under the tail in the female fetus. The difference is quite distinctive to an experienced operator. • Prior to day 53 the sex of the fetus cannot be determined because the genital tubercle is situated between the hind legs of both males and females. • After days 68–70 it may not be possible to image the whole fetus, making it harder to determine the location of the genital tubercle. Early embryonic loss is defined as the loss of the conceptus before 40 days from fertilization.56 Embryo loss can occur at any stage in the first 40 days of gestation (both before and after the earliest ultrasonographic detection). • The rates of actual fertilization in mares are estimated to be between 90% and 95% in young fertile mares bred to fertile stallions and around 80–90% in older (subfertile) mares.57 • The earliest diagnosis of pregnancy is made through ultrasonographic examination. This technology has resulted in an increase in the diagnosis of early embryonic loss. In most cases the mare has been diagnosed as ‘in foal’ at ultrasonographic and rectal examination at 12–17 days. Between 7% and 16% of equine pregnancies diagnosed by rectal examination are believed to terminate in early embryonic loss,58 but the upper limit of the range rises to 24% if diagnosis of pregnancy is made by ultrasonographic means.59 • The highest losses occur in the first 10–14 days post ovulation.60 • Losses after day 12–13 are progressively less with advancing time. • There is evidence to suggest that, in mares with twin ovulations, less than half ultimately have twin pregnancies. There is little information on the extent of early embryonic loss in the various breeds. Many causes for early embryonic loss have been implicated or proposed, including: 1. Genetic factors, which are probably the most common overall cause of loss. • Chromosomal abnormalities (with a fatal genetic combination) have been a regular and understandable cause of embryonic loss.61 • Mares bred to stallions with karyotypic abnormalities (e.g. 63/X0, 65/XXY, 64/XY) show a higher rate of embryonic loss. • Late fertilization with aging sperm or an aging oocyte may also be involved. • A significant proportion of embryos will have accidental or inherited genetic abnormalities that are not consistent with life and the embryo can die at an early developmental stage. 2. Maternal management, age and health status (including nutritional status and extent of stress). • The rate of loss within the first 14 days for young healthy mares is around 25%, but in older mares this rises to 60%. • Subfertility appears to be a major factor in early embryonic loss.62 Older mares have a higher rate of embryo abnormalities and so would be expected to suffer significantly from early embryonic loss.63 • Mares bred on foal heat have higher rates of loss than those bred at the subsequent estrus. • Low body weight and a low nutritional plane prior to mating, and acute food deprivation involving significant weight loss result in increased embryonic death. Lactation may have a direct effect on the survival of the embryo or there might be an indirect effect involving nutritional stresses. • Any type of illness, injury or other stressor (e.g. chronic laminitis) is believed to be a significant cause of early embryonic loss. 3. Uterine environmental problems. • Uterine disease has long been regarded as a cause of early embryonic loss and it is reasonable to suppose that a hostile uterine environment would cause problems such as: • The effect of endometrial (lymphatic) cysts is controversial but in some cases they may cause loss. Endometrial cysts are more commonly found in older mares and are far less common in ponies. Mares with one or two cysts (even large ones) may breed normally; multiple cysts can result in embryo loss, although this may not reflect the presence of cysts.64 Large or multiple cysts may inhibit embryo mobility and thereby block maternal recognition. Alternatively, the fibrosis/nesting commonly seen in mares with multiple endometrial cysts may result in improper emptying of the lymphatics and glands resulting in improper uterine protein and a hostile environment for the embryo. • Embryo loss may be due to an abnormal uterine environment, such as that associated with chronic inflammation, uterine fibrosis and lymphatic stasis65 (Fig. 7.19). • Alterations in the hormones that are responsible for the maintenance of pregnancy (see p. 230) may be involved, but the cause of these alterations may be difficult to establish. 5. Abnormalities of embryo location. • Embryos that develop ‘upside down’ (the normal embryo develops centrally on the floor of the vesicle and gradually moves upwards were thought to be abnormal, but there is no abnormally high failure rate. • Fixation of the embryo within the body of the uterus rather than at the base of one of the horns (regardless of the location of the subsequent fetus) means that the conceptus is not palpable per rectum and may even be missed during ultrasonographic examination. The majority of these pregnancies fail. • Fixation of the embryo in the distal regions of the uterine horns is likely to fail. • An abnormally large vesicle at day 17 (often over 30 mm in diameter, and often with an abnormal or irregular shape) may be associated with embryonic loss, but some embryos certainly survive. • Small vesicles (<4 mm at day 13) that fail to reach 20 mm in diameter by day 20 commonly fail to survive.66 This may be due to failure to induce production of the pregnancy-sustaining antiluteolytic signals. In general, vesicles that on days 13–15 are 1 day or more smaller than the normal size-for-age have a high incidence of failure. Accurate measurement is therefore essential and suspicious vesicles should be re-examined at 2-day intervals. • Anembryonic vesicles are invariably small for their gestational age. Vesicles that fail to develop an embryo by day 20 or are not readily visible at day 24 (usually of a size consistent with a 21-day gestation) will inevitably fail. Thus, if an embryo is not visible by days 24–27 the pregnancy should be terminated to avoid time loss. • Abnormal accumulations of fluid can be seen around the developing embryo. • Decrease in embryo size or failure to continue to develop normally. Reduction of twin pregnancies can be a problem with respect to the remaining embryo. Following reduction of a twin embryo, the surviving embryo often continues to normal term, but there is a high rate of early loss and so continued monitoring must be undertaken. The reduction procedure is most successful if carried out during the first 14–17 days when the embryos are still mobile (see p. 245). In the vast majority of cases, twin pregnancies in the mare result from dizygotic (separate) ovulations.67 • Identical twins resulting from a single fertilized ovum are particularly rare in horses.68 • The incidence of twins is higher in certain breeds. In the Thoroughbred and Draft breeds, 16% of total ovulations will result in twin conceptions. The proportion is lower in other breeds.69 Pony mares have a relatively low incidence of double ovulation. • Multiple ovulations are more common in older mares than in younger mares and are also more common within 80 days of foaling. • Mares that tend to double-ovulate will usually do so over multiple subsequent cycles and from one year to the next, and they therefore remain prone to twin pregnancy. The ovulations may occur within 24 hours of each other (synchronous ovulation), or more than 24 hours apart (asynchronous ovulation).70 • The twins may fix in the same horn (unilateral or unicornual twins; Fig. 7.20) or in both horns (bilateral or bicornual twins; Fig. 7.21). Fig. 7.20 The outcome of unicornual twin pregnancy (the twin embryos are implanted in the same uterine horn) in the mare. In spite of up to 70% of twin pregnancies being unicornual, only 1% will result in natural full-term births. Fig. 7.21 Outline of the incidence and distribution of bicornual twin pregnancy (the embryos are fixed in different uterine horns) in the mare. Only 30% of twin pregnancies are bicornual; of these, the large majority will produce only one full-term foal or will be barren. • If the ovulations are synchronous, the vesicles appear to be of similar size on ultrasound examination, whereas for asynchronous ovulations the vesicles may be significantly different in size.
PREGNANCY
EARLY EVENTS
Maternal recognition
ENDOCRINOLOGY OF PREGNANCY26
Equine chorionic gonadotropin (eCG)
Progesterone and progestins during pregnancy
Estrogens
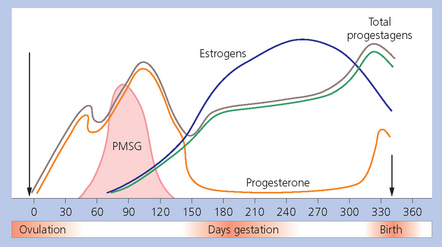
Summary of hormonal events of pregnancy
Equine chorionic gonadotropin (eCG)
Progestins (progestogens)
Estrogens
DIAGNOSIS OF PREGNANCY
Absence of estrus
Rectal examination (see Table 7.2)
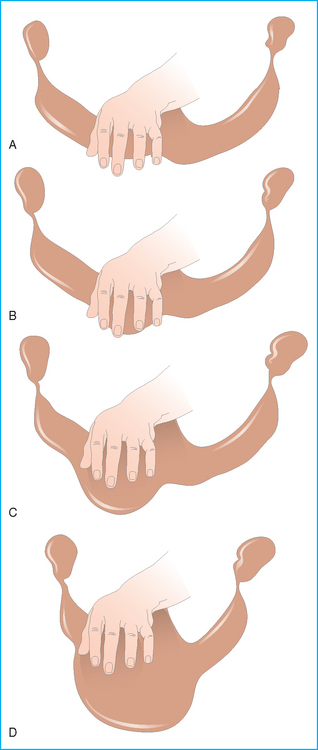
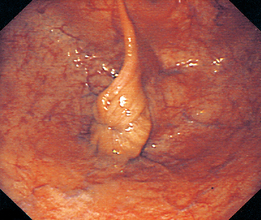
Vaginal examination
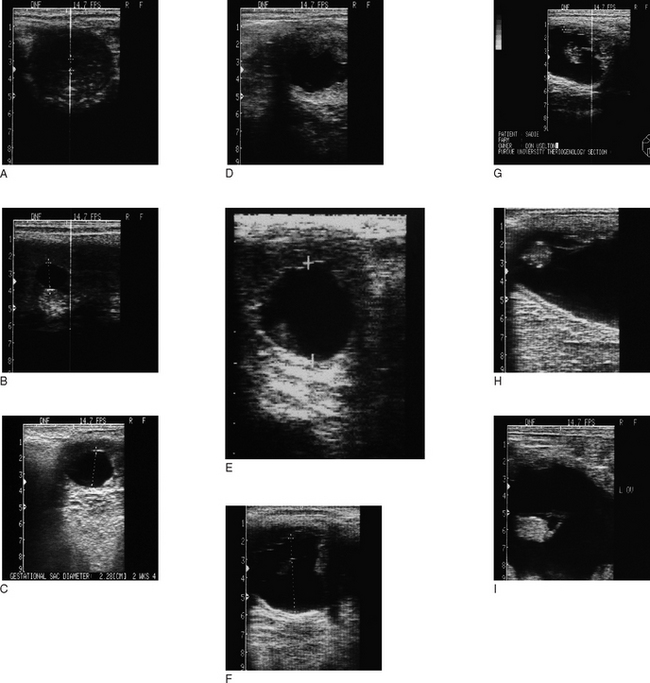
Ultrasonographic examination (see Table 7.2; Fig. 7.14)
 The sex of the foal can be confirmed at around days 55–75, and sometimes much later.
The sex of the foal can be confirmed at around days 55–75, and sometimes much later.
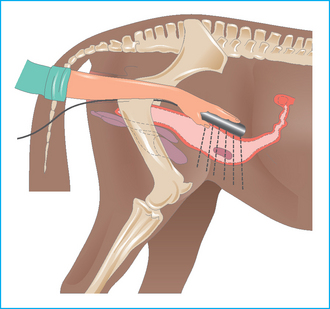
 The age of the fetus can be established by reference to standard measurements for orbital diameter, etc. If the date of service is known, the maturation and growth status of the fetus can be established. Retarded growth can be established early and the pregnancy can be classified as high risk.
The age of the fetus can be established by reference to standard measurements for orbital diameter, etc. If the date of service is known, the maturation and growth status of the fetus can be established. Retarded growth can be established early and the pregnancy can be classified as high risk.
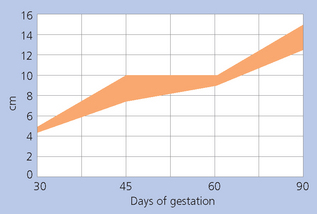
 The volume and nature of fluid surrounding the conceptus can also be calculated by ultrasonographic measurements, which is more useful in late-term pregnancy (see Fig. 7.18).
The volume and nature of fluid surrounding the conceptus can also be calculated by ultrasonographic measurements, which is more useful in late-term pregnancy (see Fig. 7.18).
Hormone tests
Progesterone concentrations
eCG concentrations
Estrogen concentrations
Summary of pregnancy diagnosis
Days of gestation
Accuracy
Ultrasonography
14 to term
Very high
Rectal palpation
18–20 to term
High
Nonreturn to estrus
17–21 post ovulation
Low to moderate
Increased progesterone
17–21 post ovulation
Moderate
Increased equine chorionic gonadotropin
45–120
Moderate to high
Total estrogens
90–100 to term
Moderate to high
 Diagnose pregnancy accurately by days 14–16 post ovulation.
Diagnose pregnancy accurately by days 14–16 post ovulation.
 Diagnose a twin pregnancy in a very early stage.
Diagnose a twin pregnancy in a very early stage.
 Accurately determine the age of the pregnancy if accurate breeding dates are not available.
Accurately determine the age of the pregnancy if accurate breeding dates are not available.
 Monitor fetal and placental development, with early determination of abnormalities occurring in the pregnancy.
Monitor fetal and placental development, with early determination of abnormalities occurring in the pregnancy.
ASSESSMENT OF THE FETUS
DETERMINATION OF FETAL SEX
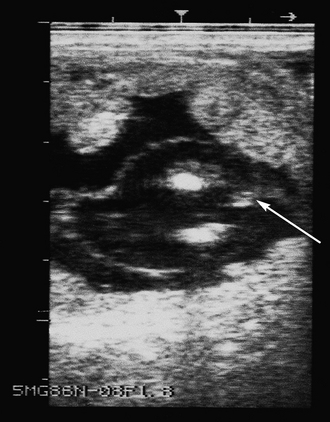
EARLY EMBRYONIC LOSS
Etiology
 Infection or other inflammation.
Infection or other inflammation.
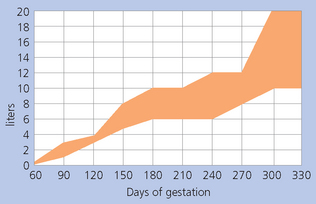
 Accumulated intrauterine fluid (including lochia and inflammatory fluid or exudate) (Fig. 7.18).
Accumulated intrauterine fluid (including lochia and inflammatory fluid or exudate) (Fig. 7.18).
Diagnosis
Management
TWINNING
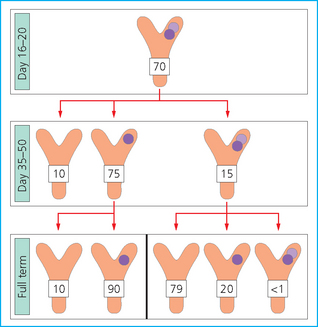
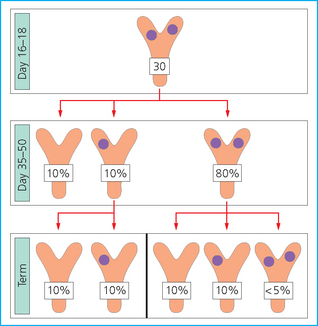
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


PREGNANCY