CHAPTER 50 Pneumocystis Infections
Pneumocystis carinii is a pathogenic fungus that most frequently causes lung disease in the immunocompromised host. Because P. carinii is an uncultivable agent, its biology and life cycle and concepts of general pathogenicity have been difficult to elucidate. Recent molecular advances have improved knowledge of P. carinii pathogenesis in immunodeficient and immunologically normal individuals.1 These observations may lead to a better understanding of P. carinii– induced disease in the horse.
ETIOLOGY
Pneumocystis organisms are a group of pathogenic fungi, some of which are host adapted.1 The organism was initially classified as a parasite closely related to the trypanosomes because of the presence of several morphologic forms and similarities in life cycle.2,3 There is a thin-walled, “trophozoite-like” form with a single nucleus, as well as a typical thick walled, cystlike organism with multiple (usually eight) inner bodies. The procyst is considered either a product or a subtype of the trophozoite stage. Knowledge of the life cycle of Pneumocystis has been determined by electron microscopy; the organism cannot be cultured in vitro, and the complete natural history of Pneumocystis remains elusive. At present, Pneumocystis is evolutionarily placed between the sister groups of fungi, Ascomycota and Basidiomycota.4 The Ascomycota are the very large group of fungi that vary from the sexual Saccharomyces to the asexual Aspergillus and Candida. The Basidiomycota include the edible and inedible mushrooms, rusts, and pathogens such as Cryptococcus.
In the absence of the ability to culture Pneumocystis in vitro and confirm species by cross-mating of isolates, a trinomial nomenclature evolved in which forma specialis (f. sp.) is used to designate each type or variety until formally designated. Sequence analysis of mitochondrial deoxyribonucleic acid (DNA) now clearly demonstrates heterogeneity between isolates and the presence of multiple species of Pneumocystis.5–7 Pneumocystis carinii f. sp. hominis is now recognized as Pneumocystis jirovecii and is thus far the only Pneumocystis species that has been found in humans.8 Each “type” or species demonstrates variability between isolates, facilitating molecular epidemiologic studies of spread and persistence of strains.8,9 The human genosequence Pneumocystis has never been demonstrated in nonhuman primates. The primate sequences demonstrates more variability than what appears to be present in the human-adapted species.9 Pneumocystis carinii f. sp. carinii, or P. carinii, is the host-adapted species for the rat.10 A specific subtype associated with equine infection was identified by sequencing of the mitochondrial ribosomal ribonucleic acid (rRNA) gene. Samples from four foals were sequenced and demonstrated 85%, 84%, and 78% agreement with human and ferret, SCID-mouse, and rat sequences, respectively.11 Species identification and alignment are an ongoing process as the genomic analysis of this organism proceeds.
EPIDEMIOLOGY
Historically P. carinii infection and disease have been considered a problem primarily in immunocompromised hosts. However, recent evidence supports the hypothesis that this organism is either a component of normal respiratory flora or a primary upper respiratory pathogen of immunocompetent animals.1,12 Seroepidemiologic studies show a positive correlation between age and presence of serum antibodies in humans.13 Exposure to the organism is apparently widespread, indicating that it is ubiquitous in the human environment.
Based on genetic studies, it is now recognized that human exposure and infection with Pneumocystis occurs during childhood, often during the neonatal period.14 Vaginally delivered rat pups are DNA positive by oral swab after delivery, indicating lateral transmission soon after birth (as from environmental and maternal respiratory secretions).15 Upper respiratory secretions of women are more likely to be DNA positive by the third trimester of pregnancy.16 In serologic studies, between 70% and 85% of children demonstrate seroconversion to Pneumocystis during childhood.17,18
The syndromes associated with Pneumocystis infection in immunocompetent children include asymptomatic infection, mild upper respiratory infection, and bronchiolitis. Several studies suggest an association between this organism and sudden infant death syndrome.19–22 Because these associations were made using molecular methods, questions remain regarding the causal relationship between Pneumocystis spp. infection and primary disease in the immunocompetent host. The presence of this organism may be related to an underlying disease process not yet identified.
With genetic sequencing, carriage of Pneumocystis in samples from the upper respiratory tract or by bronchoalveolar lavage (BAL) has been confirmed in asymptomatic animals and people with no identifiable immunologic risk.16,23–25 In one study, 20% of asymptomatic, immunocompetent people carried Pneumocystis in their respiratory tract.24 Similarly, 24% of health care workers treating human immunodeficiency virus (HIV)–infected patients are Pneumocystis carriers.25 Subtle differences in these populations that might affect immune status may still be identified. For example, some of these carriers may have chronic lung disease. Pregnancy, concomitant medications (not classified as immunosuppressive agents), or undiagnosed immune compromise may have affected the frequency of detection of Pneumocystis DNA in these studies. Asymptomatic Pneumocystis infection has also been demonstrated in mice, rats, and ferrets.23,26–28
Evidence from environmental, animal, and human molecular epidemiologic studies suggests that the fulminant syndrome, Pneumocystis carinii pneumonia (PcP), may not be the result of reactivation of a latent infection that was acquired earlier in life, as previously believed.29 Environmental monitoring casts doubt on whether Pneumocystis can propagate independent of a host. Clustering of infections has been reported since World War II. Initial infections likely originate from direct transmission of the organism from an immunocompetent carrier to an immunocompromised individual.30 Transmission of Pneumocystis genotypes occurs from HIV-infected mothers to their children, as does lateral transmission from immunocompromised patients to normal people.7,18,26,27,30–33 Air samples from hospital rooms of HIV-infected patients demonstrate identical strains of organisms.34 These types of investigations on horse farms would be of value to determine the epidemiology of PcP in equids.
Pneumocystis infections have been detected in many other animals. Immunodeficient dogs die from overwhelming Pneumocystis infections, and the organism has been detected sporadically in dogs with interstitial pneumonia.13,15,35–46 The organism has been detected sporadically in dogs with interstitial pneumonia. Young swine develop a fatal interstitial pneumonia and can be concomitantly infected with P. carinii.40,43,47–49 Cattle, goats, and sheep have reportedly been infected with P. carinii; infected goats and sheep are likely to have a pneumonitis type of disease.4,43
PATHOGENESIS
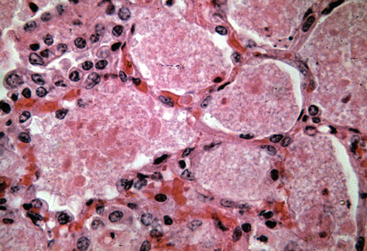
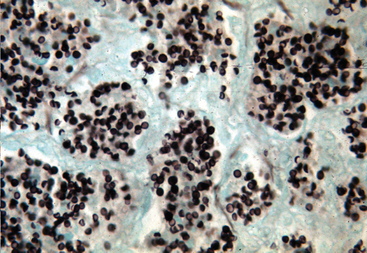
Pneumocystis infection is associated with two disease conditions in horses: acute, fulminant PcP and secondary infections with pulmonary fibrosis (pneumocystosis).50 When an overwhelming alveolar infection occurs in the immunocompromised host, the ensuing PcP is life–threatening, and the organism is readily found in the alveoli of the lung.47,50–52 It resides extracellularly in alveolar spaces, adhered to alveolar type I cells, and causes a severe exudative disease process. The tissue reaction is moderate and characterized by lymphocyte and plasma cell localization within the alveoli and mild thickening of the interstitium surrounding the alveoli. Foals with severe combined immunodeficiency (SCID) develop classical PcP in which alveoli are packed with P. carinii (Figs. 50-1 and 50-2). The alveolar spaces become moderately thickened with inflammatory cells. Severe accumulations of proteinaceous debris create dyspnea and hypoxia.
Pneumocystis infections in immunocompetent humans and horses with normal numbers of CD4+ T cells have been described. However, the requirement of CD4+ T cells for protection against disease has been demonstrated in murine models and in human patients with acquired immunodeficiency syndrome (AIDS).7,13,57–59 Loss of CD4+ cells and susceptibility to disease can be reversed by interferon-gamma (IFN-γ).60,61 Cytotoxic CD8+ T cells are an end-effector cell that mediates clearance after antigen-specific activation by CD4+ helper cells. Nonspecific CD8+ effector cells actually increase lung injury.62 Macrophages are considered important in the killing of Pneumocystis organisms; however, these cells must be augmented by T cells, IFN-γ, and granulocyte colony-stimulating factor (G-CSF).63 Other effector molecules are likely also important and have yet to be identified.
Localization of Pneumocystis to the alveoli results in accumulation of a proteinaceous fluid, causing severely impaired oxygenation.64 This condition has been compared to a disease called alveolar proteinosis. Pulmonary alveolar proteinosis is characterized by insidious onset of exercise intolerance and dyspnea. The proteinaceous material is thought to be alveolar secretions consistent with surfactants. In idiopathic disease, lack of clearance rather than overproduction of proteinaceous fluid is considered the pathogenesis. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is necessary for activation of pulmonary macrophages and removal of the pulmonary surfactant. The exact pathogenesis of proteinaceous accumulations in PcP is not known.
In many animals and humans with signs or symptoms of pulmonary disease, Pneumocystis organisms have been detected with BAL. Many of these reports describe the presence of chronic fibrosing lung pathology.33,50 In these cases, Pneumocystis may not be the inciting cause of pulmonary disease53–56 but may have colonized the compromised lung secondary to another disease process. This condition is referred to as pneumocystosis to differentiate it from acute, overwhelming PcP as previously described. Pneumocystosis in immunocompetent humans and rabbits has been associated with severe malnutrition.7,65,66
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree