CHAPTER 1 Physiology and Endocrinology of Stallions
ANATOMY AND PHYSIOLOGY
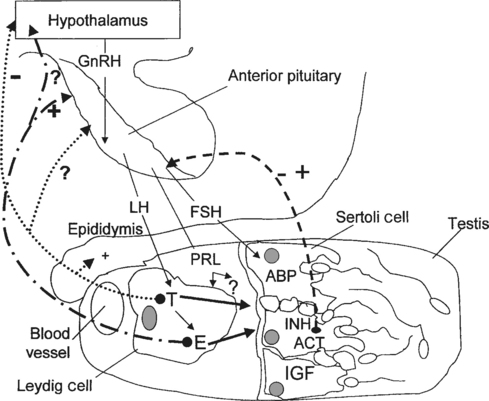
Normal spermatogenesis in stallions is a complex event, requiring a functional hypothalamic-pituitary-testicular (HPT) axis and a normal testicular environment capable of producing and supporting specific regulators of spermatogenesis (Fig. 1-1). Briefly, the anatomic features involved in the reproductive physiology of stallions include the pineal gland, hypothalamus, pituitary gland, and testis. For a detailed review of reproductive anatomy and spermatogenesis, the reader is referred elsewhere.1,2
The pineal gland is located between the cerebral hemispheres and lies dorsal to the pituitary gland. This gland produces the hormone melatonin in response to visual light signals it receives from the retina. In long-day breeders such as horses, an increase in duration of light exposure results in a decrease in melatonin production by the pineal gland. Melatonin is released in the greatest quantities during the hours of darkness. High levels of melatonin have the effect of lowering levels of gonadotropin-releasing hormone (GnRH), possibly through an influence of the feedback mechanisms exerted by androgens on GnRH release.3 Located at the base of the brain, the hypothalamus produces GnRH in a pulsatile manner in response to a variety of stimuli. Olfactory, tactile, auditory, and visual signals alter the production of GnRH by the hypothalamus, which is transported via portal vessels to the anterior pituitary gland. Pulsatile secretion of GnRH stimulates the production and pulsatile release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the pituitary.
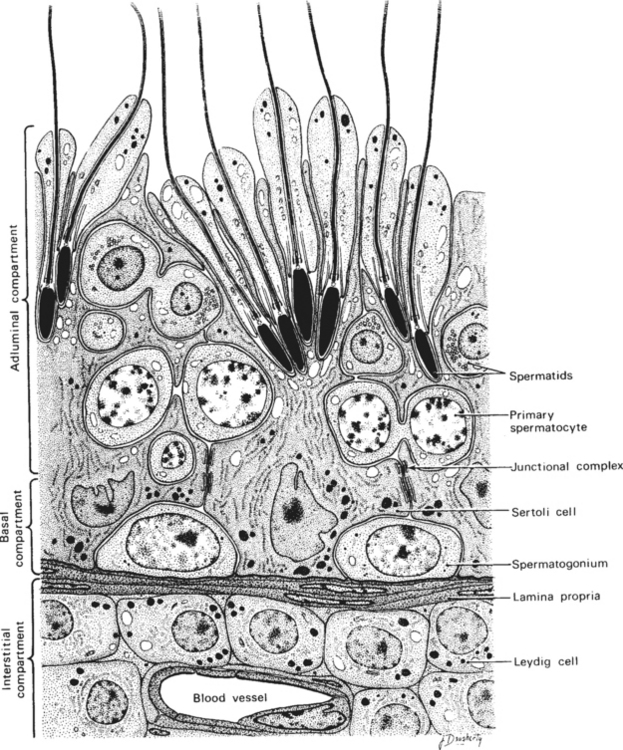
Within the testis, the seminiferous tubules of the adult stallion make up about 70% of the testicular parenchyma. The tubules consist of Sertoli cells and germ cells in various stages of development. The Sertoli cell is both a structurally and physiologically supportive cell to the process of spermatogenesis. As shown in Figure 1-2, developing sperm cells are embedded into, or in intimate contact with, the Sertoli cells throughout maturation. Most of the remainder of the testis consists of Leydig cells, myoid cells, and blood vessels of the interstitial compartment.
In adult stallions, pituitary LH binds to Leydig cell receptors, stimulating the production and release of estrogens and testosterone by the cells. Pituitary FSH stimulates the production and release of multiple factors from the Sertoli cells of the testis, including inhibin, activin, androgen-binding protein (ABP) and insulin-like growth factor (IGF). These testicular hormones and proteins feed back to the hypothalamus to regulate the pulsatile release of GnRH and therefore the continued production of LH and FSH. Estrogens, not testosterone, produced by the Leydig cells appear to regulate LH release from the pituitary gland of the stallion, either at the level of the hypothalamus by altering GnRH pulse amplitude and frequency, or directly at the level of the pituitary.4 Treatment of long-term geldings with estradiol resulted in increased LH levels and decreased FSH levels, suggesting that estrogens may also play a role in modulating FSH secretion.5 Because testosterone, inhibin, and FSH levels follow similar season patterns, with levels of all three hormones highest during the breeding season and lowest in the nonbreeding season, it has been suggested that inhibin may act together with testosterone to regulate FSH secretion by the pituitary.6 Testosterone also exerts a negative feedback mechanism on hypothalamic production of GnRH. Normal function of these many complex feedback mechanisms is critical if spermatogenesis is to proceed normally.
Androgens synthesized by the Leydig cells under the influence of LH diffuse into the blood and lymph surrounding the seminiferous tubules, becoming bound by ABP. In order for normal spermatogenesis to occur, high local concentrations of androgens, especially dihydrotestosterone (DHT), must bathe the tubules. Junctional complexes between adjacent Sertoli cells form the functional blood-testis barrier, ensuring a specific physiologic environment for the developing germinal cells on either side. The blood-testis barrier also serves to isolate diploid spermatogonia and spermatocytes from the haploid postmeiotic spermatocytes, spermatids, and spermatozoa. Myoid cells lie within the lamina propria of the tubule, just beneath the basal compartment. Containing actin filaments within their cytoplasm, these cells play a role in transport of spermatozoa toward the lumen of the tubule. The paracrine factors transforming growth factor (TGF-β) and PmodS, produced by the myoid cells under testosterone influence, may regulate Leydig cells through ABP actions and modulate Sertoli cell functions.6,7 Myoid cells may also contribute to the stability of the blood-testis barrier.8 Recently, the degeneration and transformation of myoid cells into fibroblasts was demonstrated in the testes of a group of aged (23–24 years) stallions.9 These findings suggest that myoid cells may play a significant role in the progression of testicular fibrosis in aged stallions and perhaps also in stallions with testicular degeneration.
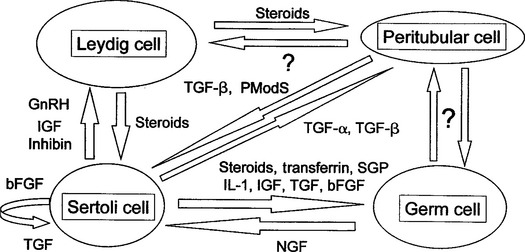
PARACRINE AND AUTOCRINE FACTORS
A paracrine factor is a product of a cell that acts locally on another nearby cell to modulate functions. An autocrine factor is a cell product that acts directly back on the cell that produced it. Testosterone, TGF-β, IGF, inhibin, and activin are some of the paracrine factors produced by the Sertoli cell that are known to regulate spermatogenesis in other species (Fig. 1-3).10 A growing body of evidence suggests a strong role for paracrine-autocrine factors in the initiation and maintenance of spermatogenesis in stallions as well. In a recent study of stallions aged 6 months to 23 years, plasma and testicular levels of IGF-I were significantly higher in colts younger than 2 years of age than in older stallions.11 These results suggest an involvement of IGF-I in testicular development and the onset of spermatogenesis. No significant differences in IGF-I levels were found among stallions over 2 years of age. No association of IGF-I levels with declining fertility was found. IGF-I levels were found to be higher in the breeding season than the nonbreeding season, but the authors cautioned that the separation of age and seasonal effects in the young stallions in the study made interpretation of this finding difficult. Inhibin, a glycoprotein hormone, has been shown recently to be produced by both Sertoli and Leydig cells in stallions.12,13 Although inhibin appears to act together with testosterone to regulate FSH secretion at the level of the pituitary gland, research in subfertile and infertile stallions suggests an important local autocrine role as well. Testicular inhibin concentrations decline in stallions with poor fertility before levels of other testicular hormones are altered,14 suggesting that a local defect in Sertoli cell function occurs first in stallions with declining fertility.
EFFECTS OF SEASON ON REPRODUCTIVE PARAMETERS IN STALLIONS
The effects of season and day length are not as dramatic in stallions as in mares. However, stallions do experience a circannual rhythm, with testicular size, semen production, libido, and hormone concentrations varying by season. An increase in the duration of light exposure results in decreased melatonin production by the pineal gland, leading to increased GnRH production by the hypothalamus. Increasing GnRH levels stimulate increased LH and FSH production by the pituitary and corresponding increases in testosterone, estradiol, and inhibin levels. The result is an increase in testicular size and weight, sperm production, and libido during the breeding season. The release of another pituitary hormone, prolactin, is suppressed by melatonin, resulting in high plasma levels of prolactin during the long days of the breeding season in stallions. Although the role of prolactin has not been elucidated in the stallion, work in other species has shown that together with LH, prolactin controls testicular LH receptor expression and activates androgen synthesis within the testis.15 Hypothalamic inhibition of prolactin release by the pituitary gland appears to be determined by dopaminergic systems; dopamine inhibits prolactin release in all species studied to date. An opioidergic inhibition of GnRH/LH release may be partially responsible for seasonal effects in stallions. In contrast to rams and hamsters,16 administration of the opioid antagonist naloxone induced LH release, followed by an increase in testosterone, in stallions during the nonbreeding season, but not during the breeding season.17,18 Geldings treated with naloxone during or outside the breeding season did not demonstrate any differences in LH production. Treatment of long-term geldings with testosterone demonstrated that the hypothalamic and pituitary feedback mechanisms sensitive to testosterone switch from a negative to positive effect between the nonbreeding and breeding seasons.19 It appears that the effects of photoperiod are dependent upon gonadal steroid feedback mechanisms, and the presence of gonads is required for seasonal effects on hypothalamic and pituitary hormones to be seen.
The endogenous reproductive rhythm of stallions can be only partially controlled by lighting conditions. Stallions maintained on continuous short days (8 hours of light: 16 hours of darkness) for 20 months continued a normal pattern of changes in reproductive parameters during the breeding season.20 Exposure of stallions to continuous long days (16:8), beginning in December (Northern Hemisphere) following a period of short days, results in an earlier attainment of peak reproductive characteristics than stallions exposed to normal environmental conditions, clearly demonstrating that seasonal reproductive recrudescence is photoinducible in stallions. However, stallions exposed to extended periods of long days appear to become refractory to light. Peak reproductive characteristics are not maintained indefinitely, with testicular regression occurring despite continued exposure to long days. This photorefractory state is an important consideration in the management of stallions servicing mares in both Northern and Southern Hemispheres. Such stallions should be exposed to several weeks of short days at the end of one breeding season before being exposed to the long days of the breeding season in the other hemisphere, to ensure a “resetting” of the circannual rhythm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree