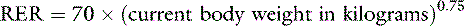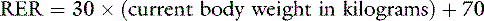Chapter 25 Parenteral Nutrition
Historical view of parenteral nutrition
Parenteral nutrition has been used routinely in human patients since the late 1960s. However, the use of parenteral nutrition goes as far back as 1656 when Sir Christopher Wren infused wine and beer into dogs using a goose quill and pig bladder.71 Although there were sporadic reports of the use of intravenous nutrition, such as the intravenous infusion of saline and milk into human cholera patients in 1832, it was not until the mid-1900s that physicians began conducting more organized experiments in providing nutrients via the intravenous route.71 Elman began administering protein hydrolysates and glucose via peripheral veins in the late 1930s and in 1947 published a book on parenteral alimentation in surgery.38 Meng and Early began using lipid emulsions in dogs in the 1940s and published an article on parenteral nutrition in dogs in 1949.38 However, it was in 1968 that physicians from the University of Pennsylvania published the seminal article on parenteral nutrition in dogs.25 These authors fed six male beagle puppies beginning at 12 weeks of age for a total of 72 to 256 days.25 The puppies were compared with their littermates that were fed orally during this same time period. The parenterally fed puppies grew at a faster rate and were larger at the end of the study compared with their littermates.25 In the same publication, Dudrick et al25 also reported on the results of feeding 30 human patients via total parenteral nutrition (TPN) for 10 to 200 days.
The Dudrick article became the start of a new era in nutritional support for hospitalized patients. After the recognition that people (and dogs) could be fed successfully for relatively long periods via the intravenous route, physicians and researchers proceeded to develop better ways to accomplish this goal. Two issues that were addressed early were developing better methods of central venous access and the formulation of parenteral nutrient admixtures. Once these techniques were further developed, a great deal of attention began to be focused on malnutrition (i.e., its prevalence, its detrimental effects, and methods for preventing and treating it). In 1976, Butterworth was the first to report an association between the mortality and morbidity associated with malnutrition in hospitalized patients.11 Soon it became widely recognized that hospital malnutrition was a major problem, which led to the concept of nutritional assessment and its role in overall patient management. In addition, clinical trials were conducted testing the benefits, complications, and timing of parenteral nutrition. In many studies, it has not been shown that routine use of perioperative TPN is justified, and often it is associated with more complications compared with enteral nutrition or no nutrition at all.8,24,29,32,70 However, certain patient populations, such as the malnourished, do appear to benefit from parenteral nutrition.8,32,70 More recently in human medicine, meta-analyses on parenteral nutrition have been conducted to determine specific patient populations in which parenteral nutrition would be most beneficial.8,29,32,64 Currently, research in parenteral nutrition in human medicine is focusing on optimizing the selection of patients who will benefit from parenteral nutrition and improved formulations, such as modified lipid and amino acid solutions.
Although dogs often have been used as models for the study of parenteral nutrition, the clinical use of parenteral nutrition in companion animal species is a relatively new modality of therapy. The first clinical report of the use of parenteral nutrition in companion animals was in 1977.12 In 1989, Lippert et al41 reported the use of TPN in seven normal cats for 14 days. In this study, cats were fed at maintenance energy requirements (MERs; calculated in that study as 1.4 × resting energy requirements [RERs]).41 However, in one group, the calories provided by protein were included in the calculations, whereas in the other group, protein calories were not included.41 Thus the latter group actually received calories in excess of MER.41 The cats that were fed more than MER developed vomiting, oral ulcerations, and hyperglycemia.41 However, all cats developed anemia, thrombocytopenia, hypertriglyceridemia, villous atrophy, and hepatocellular changes.41
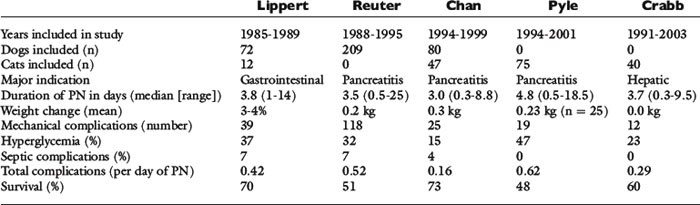
In 1993, Lippert et al43 published the first retrospective study on the use of TPN in clinical patients. This study reported the results of the use of TPN in 72 dogs and 12 cats.43 The median duration of TPN was 3.8 days, with a range of 1 to 14 days.43 Thirty-nine mechanical complications occurred, and metabolic complications were common, including hyperglycemia (37%), electrolyte abnormalities (30%), and hyperlipidemia (23%).43 Seven percent of animals developed sepsis (i.e., clinical signs of sepsis in combination with either a positive catheter tip culture or positive blood culture).43 In this study, both total calories and protein were administered at a higher level than is currently done at most institutions.43
The next major retrospective study was published in 1998 and reported the results of the use of TPN in 209 dogs.62 Similar to the Lippert study, the median duration of administration was 3.5 days (range, 0.5 to 25 days).62 One hundred-eighteen mechanical complications occurred, and 37% of the dogs had at least one mechanical complication.62 Hyperglycemia was the most common metabolic complication, with 32% of dogs evaluated developing this abnormality, but 329 individual metabolic complications occurred in this population of dogs.62 Seven percent of the animals developed sepsis.62 The overall complication rate was 0.52 complications per day of TPN.62
Two retrospective studies on the use of TPN specifically in cats also have been published.21,58 The median duration of TPN administration in these studies was 4.8 and 3.7 days, respectively.21,58 Although the number of mechanical and septic complications was similar between the two studies, 18% of cats in the Crabb et al study became hyperglycemic, compared with 47% in the larger Pyle et al study.21,58 The overall complication rate was 0.62 per day of TPN in the Pyle study and 0.29 in the Crabb study.21,58 There also were two interesting findings regarding hyperglycemia from these two studies.21,58 First, the study from Pyle et al showed that hyperglycemia was significantly associated with an increased mortality rate 24 hours after starting TPN.58 In this study, cats’ energy requirements were calculated by multiplying the resting energy requirements (RER) by an illness factor. In the Crabb et al study, cats in which the RER was multiplied by an illness factor were more likely to develop hyperglycemia than those in which energy requirements were provided at or below RER.21
Two studies have been published on partial parenteral nutrition (PPN) in clinical veterinary patients. One used a commercial three-in-one solution containing dextrose amino acids and lipid that provided 1.26 kcal/kg/hr for between 10 to 24 hr/day (n = 9) for a median of 36 hours.20 In this study, venous thrombus or thrombophlebitis was the most common cause of catheter failure.20 In a retrospective study of three-in-one PPN in 80 dogs and 47 cats, the median duration of PPN administration was 3.0 days.14 As in the previous studies, hyperglycemia was the main metabolic complication (15% of animals overall).14 Twenty-four other metabolic complications also occurred, and four septic cases were documented (3%).14 The complication rate was 0.18 and 0.15 complications per day of PPN for dogs and cats, respectively.14 One notable feature of this study is that animals that received some enteral nutrition in combination with PPN administration were more likely to survive compared with animals not receiving any enteral nutrition.14
To date, these are the most comprehensive published studies on results of parenteral nutrition use in clinical patients (see Table 25-1 for a summary of these studies). However, there are several other studies that have been helpful in enhancing the use of parenteral nutrition in dogs. The first is a study by Chandler et al19 in which an amino acid solution, a dextrose solution, and an electrolyte solution were administered to compare their effects on nitrogen balance. These solutions were individually administered to three healthy dogs via a peripheral vein for 10 hr/day for 4 days.19 Only the amino acid solution resulted in a positive nitrogen balance, suggesting that in healthy dogs it provided adequate amino acids to prevent breakdown of lean body mass.19 In 2001, Mauldin et al49 reported a study evaluating parenteral nutrition in healthy dogs. In this study, dogs received intravenous infusions of either nonlactated Ringer’s solution or isocaloric solutions containing 0, 1.36, or 2.04 g of amino acids per kilogram of body weight per day, with the remaining calories (to meet MER) provided by dextrose and lipid solutions for 12 hr/day for 7 days.49 On Ringer’s and 0 g/kg amino acids, dogs had negative nitrogen balance, and a regression analysis suggested that intravenous administration of 2.32 g/kg/day of amino acids would result in zero nitrogen balance in a healthy dog of beagle size (i.e., the minimum amount required to prevent catabolism of lean body mass by supplying basal amino acid requirements).49 Zentek et al published a study of parenteral nutrition in healthy laboratory dogs using a three-in-one solution with the majority of calories either from glucose or lipid.73 In this study, PPN was administered cyclically (over 10 hr/day) to meet MER.73 Depending on the formula (high glucose or high lipid), dogs had significant increases in blood glucose and triglycerides, respectively, but there were no significant differences in hormonal concentrations (e.g., insulin-like growth factor-1, insulin, glucagon, T3, T4).73 An in vitro study also was recently published assessing physical effects on the stability of lipid-based parenteral nutrition solutions.66 This is important information as factors such as temperature and handling can affect the nutrition and physical stability of parenteral nutrition solutions. This increasing number of studies has been helpful in better understanding the metabolism of parenteral amino acids in companion animals and will serve as a foundation on which to base future research into the specific requirements of ill and injured animals.
Patient selection
Any route of nutritional support carries some risk of complications, and parenteral nutrition is not an exception. Studies in people have shown that parenteral nutrition in some patient populations actually increases the risk of complications and worsens the outcome.* Therefore careful patient selection is particularly important in the case of parenteral nutrition. Ideally, one would select only those patients that would benefit from parenteral nutrition, but the appropriate selection criteria are not yet known in people or in companion animals. Most companion animals receive parenteral nutrition for relatively short periods (median, 3 to 4 days), and one must determine whether short-term provision of parenteral nutrition is likely to be beneficial. Occasionally, parenteral nutrition is administered for more prolonged periods, and as always the risk/benefit ratio must be considered. In a previously healthy dog that has been anorectic for 2 to 3 days and in which oral or enteral intake is likely to resume quickly, parenteral nutrition may not be beneficial. However, in a vomiting cat that has not eaten for 1 week at home and is not expected to be eating again soon, parenteral nutrition would be indicated.
The indications for parenteral nutritional support are situations in which an animal cannot voluntarily consume adequate calories and cannot tolerate enteral nutrition. The specific indications for parenteral nutrition are shown in Box 25-1. Proper patient selection is an important aspect of nutritional assessment because administration of parenteral nutritional support to patients unlikely to benefit from this form of nutrition only subjects them to risk of complications.
Nutritional assessment
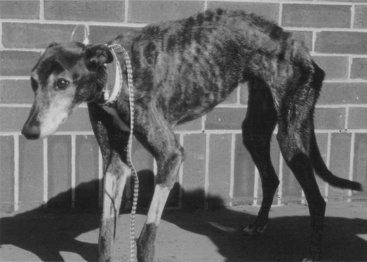
It is easy to recognize the classical picture of the starved patient as being malnourished (Figure 25-1). However, many of our patients have more subtle signs of malnutrition or develop malnutrition while hospitalized because the risk for malnutrition was not recognized early enough to prevent it (Figure 25-2, A). Even obese animals are at risk for malnutrition (Figure 25-2, B) because if they lose weight, they will lose lean body mass rather than fat. Assessment of nutritional status should be incorporated into the daily examination of each patient. Nutritional assessment identifies malnourished patients that require nutritional support and also identifies patients at risk for malnutrition in which nutritional support will help prevent malnutrition.15,53
For many years, investigators have attempted to develop a single measurement or group of measurements that will identify malnutrition in humans. Unfortunately, few of these have worked well on a clinical basis. Therefore, most nutritionists in human and veterinary medicine use a subjective global clinical assessment to identify patients in need of nutritional support (Box 25-2). This assessment includes historical information (e.g., duration of clinical signs, history of anorexia or weight loss), clinical parameters (e.g., underlying disease, degree of weight and/or muscle loss, severity of illness, clinical signs, anticipated course of recovery), and laboratory results. Any clinical or laboratory findings that would specifically alter the nutritional plan should be carefully considered. Examples include the presence of congestive heart failure (which would necessitate careful attention to fluid volume), electrolyte abnormalities, hyperglycemia, hypertriglyceridemia, or hepatic encephalopathy. These factors then are incorporated into an overall assessment of the degree of malnutrition or the animal’s risk for developing malnutrition. Prevention (or correction) of nutritional deficiencies and imbalances then can be accomplished by providing adequate energy substrates, protein, and micronutrients.
BOX 25-2 Indicators of Malnutrition in Dogs and Cats
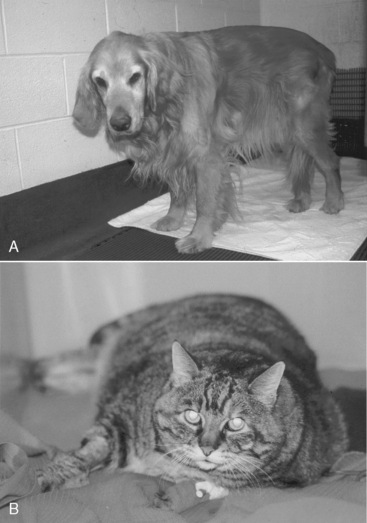
The authors categorize hospitalized animals into three groups: (1) those that are already malnourished (see Figure 25-1); (2) those that are not malnourished but are at high risk for developing malnutrition (Figure 25-3); and (3) those that are not malnourished and are at low risk for developing malnutrition (Figure 25-4). Animals in the first group require prompt nutritional support. Animals in the second group require nutritional support in the first 2 to 3 days of hospitalization, or at the time of anesthetizing them for diagnostic or therapeutic procedures, a feeding tube should be placed. Factors that put an animal at high risk for malnutrition include anorexia lasting longer than 3 days (be sure to include the time the animal has been anorectic at home before admission to the hospital), serious underlying disease (e.g., trauma, sepsis, peritonitis, pancreatitis, extensive gastrointestinal surgery), and large protein losses (e.g., protracted vomiting or diarrhea, open abdomen, or large draining wounds). Animals in the third group do not require immediate nutritional support and can be monitored to ensure adequate food intake. However, if the underlying disease does not resolve quickly or the animal continues to be anorectic, nutritional support may be required. Indicators of malnutrition are listed in Box 25-2.
Route of nutritional support
Most clinicians have heard the adage, “If the gut works, use it.” This approach still holds true, and parenteral nutrition should not be the first consideration in an ill or injured animal. The suitability of the enteral route should always be addressed first because it is the safest, most convenient, most physiologically sound, and least expensive method of nutritional support (see Chapter 26.26 If only parts of the gastrointestinal tract are functional, consideration should be given to using those functional segments. For example, a dog or cat with severe esophagitis should be considered a candidate for a jejunostomy feeding tube. However, when patients are unable to tolerate any enteral feeding, parenteral nutrition should be considered. Before parenteral nutrition is instituted, however, it is critical that fluid, electrolyte, and acid-base abnormalities are corrected.
Parenteral nutrition
Parenteral nutrition can be delivered via a central vein (TPN) or a peripheral vein (PPN). TPN, as defined in this chapter, is the provision of all of the animal’s calorie and protein requirements (and ideally, all of the micronutrient requirements as well; see Other Nutrient Requirements section). PPN only supplies part of the animal’s energy, protein, and other nutrient requirements.74 In this chapter, we use the abbreviation PPN to refer to partial parenteral nutrition, which can be supplied through either a peripheral or central vein.
Because TPN will supply all of the animal’s calorie and protein requirements, it is usually the modality of choice for an animal requiring parenteral nutrition. The disadvantages are that it requires a jugular venous catheter and it may be associated with more metabolic complications. PPN may be an alternative to TPN in selected cases (Box 25-3), but it is important to be aware that it will not provide all of the animal’s requirements. Both TPN and PPN are typically a combination of dextrose, an amino acid solution, and a lipid solution. However, the concentration of some components (e.g., dextrose) varies depending on whether TPN or PPN is chosen. TPN or PPN can also be formulated with amino acids and dextrose alone, without any lipid.9Table 25-2 compares a PPN and a TPN admixture for a 20-kg dog.
• To maintain nutritional status, rather than replete the malnourished patient. Debilitated patients should get total parenteral nutrition (TPN) or a combination of PPN and enteral nutrition.
• Animals with average nutritional requirements. Those with high requirements (e.g., open abdomen, large draining wound, severe vomiting or diarrhea) should receive TPN.
• When only short-term nutritional support in a nondebilitated patient is anticipated (<5 days).
• To supplement oral or enteral nutrition.*
Table 25-2 Parenteral Nutrition (PPN) and Total Parenteral Nutrition (TPN) Formulations for 20-kg Dog with Acute Pancreatitis
| PPN | TPN | |
|---|---|---|
| 5% dextrose (mL) | 900 | — |
| 50% dextrose (mL) | — | 164 |
| 8.5% amino acids (mL) | 450 | 312 |
| 20% lipid (mL) | 77 | 139 |
| Total mL/day | 1427 | 615 |
| PPN mL/hr | 60 | 26 |
| Maintenance fluid rate (mL/hr) | 55 | 55 |
Formulation of parenteral nutrition requirements
Calorie Requirements
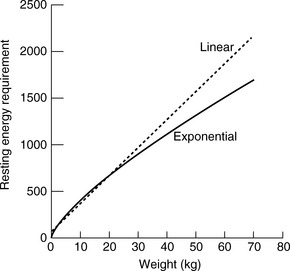
One should avoid using the linear equation for animals smaller than 3 kg or larger than 25 kg because the linear equation will underestimate or overestimate these animals’ energy requirements, respectively (Figure 25-5).
In the past, the RER was multiplied by an illness factor between 1.0 and 2.0 to account for increases in metabolism associated with different conditions and injuries.1,10,42 Recently, there has been less emphasis on these subjective illness factors, and current recommendations are to use more conservative energy estimates to avoid overfeeding.15,28 Overfeeding can result in metabolic and gastrointestinal complications, hepatic dysfunction, and increased carbon dioxide production.5,6 We have shown in cats receiving TPN that those in which the RER was multiplied by an illness factor were more likely to develop hyperglycemia than those in which energy requirements were provided at or below RER.21 Critically ill cats, in particular, are at high risk for hyperglycemia due to a variety of hormonal alterations that are similar to those identified in critically ill people.17 Avoiding the development of hyperglycemia has been shown to be beneficial in certain populations of critically ill people.27,69 Newer recommendations for nutritional support in critically ill people emphasize the need to avoid overfeeding and reduce the risk of hyperglyceamia.40,47,60 In cats16,69 and in dogs,67 hyperglycemia is associated with reduced survival and longer hospitalization times. To reduce the risk of hyperglycemia and other complications, the authors use RER as an initial estimate of a critically ill patient’s energy requirements. Further adjustments are made based on the animal’s response to feeding, body weight, and changes in the underlying condition. Indirect calorimetry can accurately assess the caloric needs of individual patients, but it is rather cumbersome to use in a clinical setting. This technique may be more commonly used in the future, particularly for patients that are difficult to manage on nutritional support. Studies using indirect calorimetry support the hypothesis that the application of illness factors in calculating energy expenditure in clinical patients overestimates energy needs.57,72 At the current time, the key to successful nutritional support is vigilant monitoring after therapy has been initiated to ensure that provision of calories is adjusted as necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree