CHAPTER 25 Papillomavirus Infections
Equine papillomavirus types 1 and 2 have been isolated from acquired papillomas (warts) and aural plaques in young horses. A large number of studies have implicated bovine papillomavirus types 1 and 2 in the pathogenesis of equine sarcoid, a form of nonmetastatic cutaneous fibrosarcoma, but a causal link has not been confirmed by fulfilling Koch’s postulates. Each of these disorders is discussed separately in this chapter.
EQUINE WARTS
Etiology
Cutaneous papillomas (warts) are proliferative skin lesions caused by infection with Equus caballus papillomavirus type 1 (EcPV-1). Lesions are most often observed on the muzzle and lips of young horses (Fig. 25-1) but may also appear on ears, eyelids, genitalia, or distal limbs.1 Papillomaviruses are small, nonenveloped, double-stranded deoxyribonucleic acid (DNA) viruses that are associated with epithelial proliferations in many vertebrate species.2 EcPV-1 has been isolated, characterized, and cloned from equine warts.3 A second mucosotropic equine papillomavirus, designated EcPV-2, has been isolated from papillomas affecting the genital area of horses. EcPV-2 differs from EcPV-1 in restriction endonuclease digestion pattern.3,4
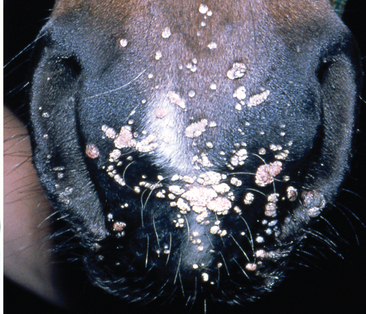
Fig. 25-1 Typical appearance of warts on muzzle and lips of young horse.
(Courtesy Dr. Melissa Hines.)
Congenital equine papillomatosis is much less common than acquired disease.5–11 Congenital lesions do not spontaneously regress, but surgery is usually curative.11 Although in utero infection by a latent papillomavirus in the dam has been suggested as an etiology of these lesions, there has been no genetic or antigenic evidence to support this.8,12
Epidemiology
Most horses with cutaneous papillomatosis (warts) are less than 3 years of age. There are no breed or gender predilections. Disease can be spread by fomites or by close contact with affected horses. Spread is common when young horses are brought together in large groups for show, sale, or breeding.1
Pathogenesis
In 1951, Cook et al.13 demonstrated that intradermal or subcutaneous injection of filtrates of equine papillomas results in growth of typical papilloma lesions within 60 to 70 days. Inoculation by skin scarification produces similar results. Lesions regressed spontaneously within 50 to 100 days. Similar injections into calves, lambs, dogs, rabbits, and guinea pigs did not result in lesions.13 Natural infections are thought to require contact of virus with damaged skin (environmental trauma, ectoparasites, ultraviolet light damage).11 The incubation period is estimated at approximately 60 days and may be influenced by the dose of virus, route of exposure, and immunity of the host.11,14
Histopathologic studies of naturally occurring equine warts suggest that lesions are initiated by basal cell hyperplasia without viral antigen production. As lesions develop, there is prominent acanthosis with cellular swelling and fusion, as well as marked hyperkeratosis and parakeratosis.15 Hypomelanosis of lesions may be related to a disturbance in melanin synthesis.16 Regression of papillomas is associated with an increase in the number of hyperfunctional Langerhans’ cells at the dermal-epidermal junction and infiltration of T lymphocytes.17 Natural immunity is strong, and warts disappear spontaneously 1 to 6 months after they initially appear.11
Clinical Findings
Horses with equine cutaneous papillomatosis usually have multiple lesions, most frequently on the muzzle and lips but occasionally occurring on the distal limbs, genitalia, ears (Fig. 25-2), and eyelids.1,11 Equine warts vary in size from 0.1 to 2 cm in diameter. When they first appear, warts are often slightly raised, flat, smooth, and flesh colored. As they proliferate in number and size, they become verrucous, gray, and hyperkeratotic with development of fronds, resulting in a cauliflower-like appearance.1 Single or multiple lesions may be oval or irregular in outline, dry, gray or red in color, and with rough, wrinkled, or smooth surfaces. Depigmentation may occur as warts slough.
Pathologic Findings
Biopsy of papilloma lesions reveals epidermal hyperplasia and papillomatosis without connective tissue proliferation. Ballooning degeneration of epidermal cells, clumping of keratohyaline granules in the cytoplasm, and basophilic intranuclear inclusion bodies may be observed.1,11,15
Therapy
Most horses with warts do not require treatment. If removal is necessary for immediate esthetic enhancement, cryosurgery with a two-cycle, freeze-thaw-freeze technique is a valid treatment. Chemical cautery with trifluoroacetic acid is also considered safe and effective. A solution of 25 g anhydrous trifluoroacetic acid, 3 g water, and 20 g glacial acetic acid is applied to affected tissues only. Adjacent tissues should be protected with petroleum jelly. Applications are repeated on the fourth and seventh days after initial treatment.1 Topical treatment with podophyllin (50% podophyllin; 20% podophyllin in 95% ethyl alcohol; 2% podophyllin in 25% salicylic acid) and undiluted medical-grade dimethyl sulfoxide (DMSO) may be used once daily until remission occurs. There is no scientific evidence to support the theory that surgical excision of some lesions enhances or hastens regression of remaining lesions. Recurrence after surgical excision has been reported.1,11 A variety of immunomodulatory drugs, including mycobacterial cell wall extracts and Propionibacterium extracts, have also been recommended for treatment of equine warts.
Prevention
Affected horses should be isolated from uninfected animals. Skin trauma from the environment or ectoparasites should be minimized. Disinfection of premises and equipment with formaldehyde or lye is recommended after exposure to a horse with warts.1,11
AURAL PLAQUES
Etiology and Pathogenesis
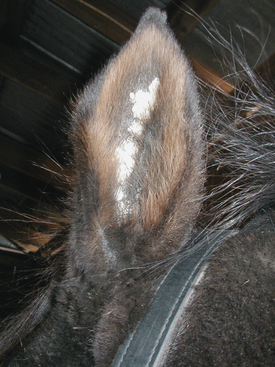
Aural plaques (papillary acanthoma, hyperplastic dermatitis of the ear) are raised, depigmented lesions on the inner surface of the pinnae of the ear.11,18–20 Papillomavirus has been demonstrated in these lesions by electron microscopy (EM) and immunohistochemistry (IHC).21 The pathogenesis of aural plaques in horses is unclear. Lesions often appear worse in the summer and fall, possibly because of fly irritation.
Clinical Findings
There are no age, breed, or gender predilections for occurrence of aural plaques in horses.11 Lesions are often bilateral and progress from small, smooth, depigmented papules and plaques to larger, often coalescent, hyperkeratotic plaques (Fig. 25-3). Aural plaques do not spontaneously regress, and they may be more active in the summer, possibly in association with black fly bites. Plaques are not sensitive, pruritic, or painful but may be considered esthetically displeasing by owners. Similar lesions may occur around the anus and vulva.11,22
Diagnosis
The diagnosis of aural plaques is usually made on the basis of clinical signs. Biopsy is usually not indicated, but if performed, lesions appear histologically identical to verruca plana, a wartlike disease of humans, with epithelial proliferation and epidermal hypomelanosis.22
SARCOIDS
Etiology
A viral etiology for equine sarcoids was postulated as early as 1936 by Jackson.23 In 1948, Olson demonstrated that intradermal inoculation of horses with cell-free extracts from bovine skin tumors containing bovine papillomavirus (BPV) caused lesions resembling equine sarcoids.24,25 Ragland et al.26 described an epizootic of equine sarcoid in 1966 and confirmed the ability to induce sarcoidlike lesions in horses by inoculation with BPV in 1969.27,28 Lesions regressed spontaneously, and inoculated horses developed a humoral immune response to BPV. Subsequent studies by a variety of investigators have demonstrated the presence of DNA and ribonucleic acid (RNA) from BPV types 1 and 2, or closely related viruses, and expression of the BPV types 1 and 2 major transforming protein, E5, in equine sarcoids.29–46 BPV DNA can be detected from normal skin of horses affected with equine sarcoid and, to a lesser extent, on the skin of unaffected horses, suggesting the possibility of viral latency.32,34,41,47
Papillomaviruses are small, nonenveloped viruses with icosahedral symmetry, 72 capsomers, and a double-stranded, circular DNA genome. They are classified on the basis of their species of origin and the degree of homology with other papillomaviruses isolated from the same species.48 Most papillomaviruses infect epithelial cells, causing proliferative lesions described as warts, papillomas, or condylomas; however, some types infect fibroblasts with resultant fibroepithelial tumors. Lesions induced by papillomaviruses are usually benign and self-limiting. Some papillomaviruses are oncogenic; in particular, human papillomavirus (HPV) types 16 and 18 are associated with cervical carcinoma.36 All the open reading frames (potential coding regions) of the viral genome are located on a single strand of viral DNA. The early region of the genome encodes proteins necessary for cell transformation (E5, E6, and E7) and replication and transcription regulatory proteins E1 and E2. The late region codes for structural capsid proteins (L1 and L2). The early and late gene regions are separated by a regulatory region containing the origin of replication and many of the control elements for viral transcription and replication.48
Bovine papillomaviruses are the causative agents of bovine warts. At least six distinct bovine viruses are associated with either fibropapillomas or papillomas. The group of viruses associated with bovine fibropapillomas (BPV types 1, 2, and 5) are most often identified in association with equine sarcoids.49,50 Sequence analysis of BPV DNA from equine sarcoids suggests the possibility of equine sarcoid-specific variants of BPV.44,45
Despite the strength of evidence linking BPV with equine sarcoid lesions, there is no evidence of a productive infection with release of infectious progeny virions in horses. Viral DNA exists episomally in equine cells and does not integrate in the host cell genome.39,51 This conclusion is supported by the lack of detectable antibodies to BPV in naturally affected horses, the inability to transmit sarcoids experimentally using equine tissue extracts, and the inability to identify viral particles in sarcoid lesions using EM or immunoperoxidase staining.30,52
Bovine papillomavirus is very resistant to physical and chemical inactivation.32 It remains viable after 30 minutes at 67° C (152.5° F), is stable at a pH between 4 and 8, is stable in ether, and survives in 50% glycerol when frozen or lyophilized.53 These properties suggest that BPV may be able to survive for a long time in the environment.32
Suggestions that other viruses may be causally related to equine sarcoid are unsubstantiated. A retrovirus identified in a cell line originating from an equine sarcoid was determined to be an endogenous virus that was unrelated to the sarcoid.54,55 Equine cutaneous papillomaviruses, the etiologic agent of equine warts, are not causally related to equine sarcoids.3
Epidemiology
Equine sarcoids are the most common dermatologic neoplasm in horses, donkeys, and mules worldwide, accounting for an estimated 20% of all equine tumors56 and as many as 90% of all skin tumors.57 The incidence of sarcoid tumors in the general population of horses is not known. Sarcoids accounted for 0.7% of all equine cases presented to the Cornell University Veterinary Hospital between 1975 and 1987 and to the Ohio State University between 1976 and 1985.58
Sarcoid tumors most frequently develop when horses are 3 to 6 years of age, but lesions may occasionally be observed in yearlings.58 Equine sarcoids appear less likely to develop in horses after 7 years of age.59–62 Young male equids are at increased risk of disease.45,61,63,64
No evidence indicates that castration increases the risk of paragenital sarcoids in donkeys.63 In cool northern climates, sarcoids are most often observed on the head and abdomen of horses; in warmer climates the limbs are more frequently affected.58 It remains uncertain whether contact with cattle is a risk factor for equine sarcoids.23,64 However, a donkey living in close contact with affected donkeys is more likely to develop sarcoid tumors than the average donkey.65
Evidence for genetic predisposition for development of sarcoid tumors includes varying disease prevalence in specific breeds,64,66,67 increased incidence of lesions in some equine families,26,68,69 and an association between sarcoid susceptibility and the major histocompatibility complex (MHC)–encoded class II allele ELA W13.67,70–72 Sarcoids may occur in any breed of horse, but they are seen more frequently in Quarter Horses and less frequently in Standardbred horses than in the general equine population.64,66,67 Several studies have shown that the frequency of the ELA W13 allele in horses with sarcoids is high regardless of breed. However, the majority of horses with the W13 allele never develop a sarcoid tumor, suggesting that environmental factors (most likely BPV) and other genetic factors are also important for disease development.
Pathogenesis
The manner by which BPV induces equine sarcoid lesions is uncertain. Hypotheses include transmission of virus by direct or indirect contact with infected horses and cattle and transmission by insects.36,65,73 Although the physical properties of BPV suggest that it might be capable of surviving for a long time in the environment, extensive environmental surveys have not been performed. One study was unable to identify BPV DNA in the environment of affected and healthy horses, except for the environment of one horse living in contact with a cow with warts.32 Genetic sequences specific for BPV have been identified from skin of normal horses with no sarcoid lesions and from normal skin of horses with sarcoid tumors, suggesting the possibility of viral latency.32,34,41,47 Nucleic acid from BPV cannot be demonstrated in lymphocytes, liver, spleen, or lymph nodes of affected horses.44,74,75
Regardless of the manner of initial contact with BPV, it is clear that BPV alone is insufficient to induce sarcoid lesions in horses. The immunologic status of the horse, genetic background, and skin trauma may play a role in initiation of disease.32