CHAPTER 26 Pain Assessment and Management
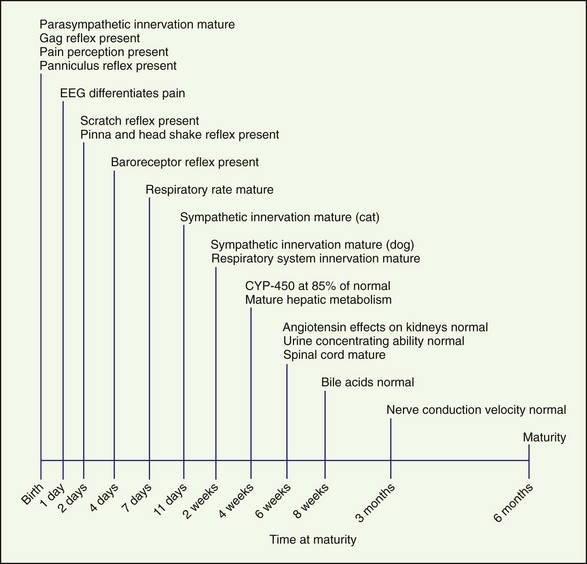
Recognition and treatment of pain in neonates is an emerging science and is controversial. To effectively treat pain in neonates, the maturity and function of many different body systems must be considered (Figure 26-1). Choosing appropriate drug therapy requires consideration of course, cause, and severity of pain and the duration and side effects of the chosen analgesic therapy (Box 26-1). In addition, other medications, concurrent medical problems and medications, and the patient’s physical status should be considered when choosing analgesics. Few clinical studies have been conducted investigating pain in neonatal dogs and cats. All too often, pain in neonates and juvenile animals is undertreated.
BOX 26-1 Clinical implications of immaturity
Physiology and Development
Development of Cardiovascular and Respiratory Systems
Tidal breathing in neonates is altered as a result of their increased chest wall compliance. The work of the neonate to move the diaphragm is greater and so is the need to generate increased negative pressure. As a result, respiratory depression that shortens the inspiratory phase negatively affects gas exchange and arterial blood gases (Box 26-2).
Drug Absorption, Metabolism, and Excretion
The kidneys are functionally and morphologically immature at birth and continue to develop for 2 to 3 weeks after birth. Glomerular filtration rate (GFR) and renal plasma flow (RPF) are lower in the neonate than the adult, and this correlates with a lower arterial blood pressure. As arterial blood pressure normalizes, GFR and RPF increase because GFR and RPF in neonates are directly correlated with arterial blood pressure. The effect of angiotensin on the kidneys does not reach maturity until 6 weeks of age. Urine concentrating ability also matures at approximately 6 weeks of age (Box 26-3).
Development of the Spinal Cord, Pain Receptors, and Pain Recognition
The initial noxious stimulus produces transduced electrical signals that are transmitted by afferent sensory neurons to projection neurons in the spinal cord, each of which has receptors that are under developmental control. In addition, these peripheral sensory neurons are overproduced during embryonic development and nearly half of these sensory neurons undergo programmed cell death in the adult. As a result, during postnatal development the perception of pain and its inhibition undergoes waxing and waning based on the neurobiologic development of the animal. This explains large interspecies and interindividual variability in the response to noxious stimuli (Box 26-4).
Physiology and Pathophysiology of Pain in Neonates
Pathophysiology of Pain
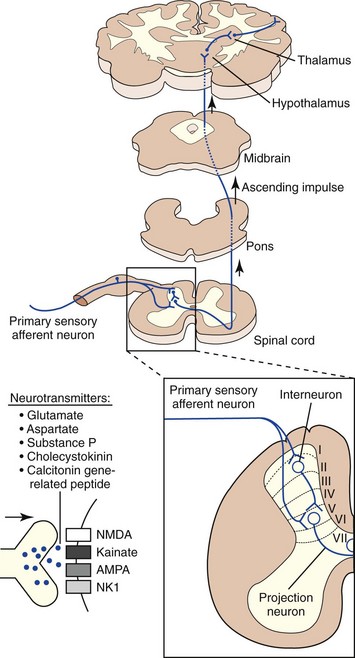
Nociceptive impulses are subjected to modulation and integration before reaching their ultimate destination, the cerebral cortex. Other supraspinal structures that process and modulate nociceptive input include the reticular formation, periaqueductal grey matter, limbic system, and the thalamus. The thalamus is the major relay station for all sensory input en route to the cerebral cortex. It is especially important for integrating nociceptive impulses and is composed of numerous complex nuclei: the lateral thalamic nuclei are involved in sensory discriminative aspects of pain, while the medial thalamic nuclei are involved in motivational affective aspects of pain. The cerebral cortex is the site where the physiologic process of nociception is integrated and ultimately perceived (Figure 26-2). Several discrete cortical regions are preferentially activated by noxious stimulation: first and second somatosensory cortices, anterior insular cortex, and anterior cingulate. Neurotransmitters in the thalamocortical region are excitatory (glutamate and aspartate) and inhibitory (gamma-aminobutyric acid [GABA], glycine, monoamines, acetylcholine, and histamine). Differences between the integration of nociceptive impulses in neonates compared with adults are unknown.
Pain Assessment in Neonates
Behavioral and physiologic parameters may be used to assess pain in neonates and are more useful in predicting stressful situations than in adults. Pain behavior in neonates and juveniles includes a change from normal behavior patterns and display of new behaviors such as positioning the painful area in such a way as to limit pressure or stimulation. In neonates, this may include a change in sleeping and eating patterns. Because this constitutes the majority of a neonate’s time, a decrease or increase in these behaviors may be indicative of pain perception. Increased vocalization (whining, whimpering, and crying) may also be an indicator of pain. Physiologic indicators of pain in neonates include changes in heart rate, respiratory rate, blood pressure, and oxygen saturation (Box 26-5).
Pain Control
Sedatives
Acepromazine
Caution should be used when administering acepromazine to animals that are predisposed to seizures or with a seizure history because it can lower the seizure threshold. The consequences of this effect remain speculative. The dose of acepromazine should be decreased in neonates or those animals with hepatic insufficiency caused by its slower metabolism and potentially long duration. Acepromazine is a safe and effective tranquilizer in juvenile animals (Table 26-1).
TABLE 26-1 Drugs useful for treating pain in neonates
| Drug | Dose |
|---|---|
| Phenothiazine Tranquilizers | |
| Acepromazine | 0.01-0.05 mg/kg IM, SC |
| Benzodiazepines | |
| Diazepam | 0.1-0.4 mg/kg IV |
| Midazolam | 0.1-0.4 mg/kg IV, IM |
| Alpha-2 Agonists | |
| Medetomidine | |
| Xylazine | 1-2 mg/kg IM |
| Opiates | |
| Morphine | 0.2-1 mg/kg IM |
| Oxymorphone | 0.02-0.2 mg/kg IV, IM, SC |
| Hydromorphone | 0.02-0.2 mg/kg IV, IM, SC |
| Fentanyl | 0.002-0.004 mg/kg IV, IM |
| Fentanyl patches | |
| Meperidine | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|