pleural cavity, mediastinum, thoracic oesophagus, thoracic wall
CHAPTER CONTENTS
Pleural cavity199
8.1 Anatomy and radiography of the pleural cavity199
8.2 Increased radiolucency of the pleural cavity200
8.3 Increased radiopacity of the pleural cavity202
8.4 Pleural and extrapleural nodules and masses204
8.5 Ultrasonography of pleural and extrapleural lesions205
8.6 Pleural thickening: increased visibility of lung lobe edges205
Thoracic oesophagus213
8.14 Normal radiographic appearance of the thoracic oesophagus213
8.15 Oesophageal contrast studies: technique and normal appearance214
8.16 Abnormalities on oesophageal contrast studies214
8.17 Oesophageal dilation215
8.18 Variations in radiopacity of the oesophagus217
8.19 Oesophageal masses217
8.20 Oesophageal foreign bodies218
Thoracic wall219
8.21 Variations in soft tissue components of the thoracic wall219
8.22 Variations in the ribs220
8.23 Variations in the sternum221
8.24 Variations in thoracic vertebrae222
8.25 Ultrasonography of the thoracic wall222
8.26 Variations in the appearance of the diaphragm222
8.27 Ultrasonography of the diaphragm224
PLEURAL CAVITY
8.1. ANATOMY AND RADIOGRAPHY OF THE PLEURAL CAVITY
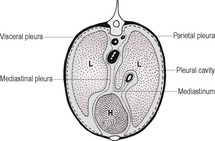
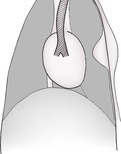
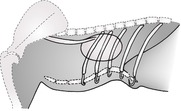
The pleural cavity is a potential space between visceral and parietal pleura and surrounding each lung (Fig. 8.1). It contains only a small amount of serous fluid and is normally not visible. Visceral pleura is adherent to the lung surfaces; parietal pleura lines the thoracic wall and forms the mediastinum. In the dog, the caudoventral mediastinal pleura has fenestrations connecting the right and left pleural cavities, making bilateral pleural disease more likely. In the cat, the mediastinal pleura is often intact and unilateral pleural disease is more common. Unilateral or asymmetric pleural pathology may result in a mediastinal shift, with the heart and associated structures moving to the opposite side (see 8.8).
Radiography for suspected pleural disease should always include orthogonal views (lateral and dorsoventral, DV, or ventrodorsal, VD). Positional radiography (e.g. VD versus DV, horizontal beam views) may be helpful to distinguish pleural pathology from other thoracic pathology and to tell whether fluid is freely moving or trapped, provided that placing the animal in other positions does not compromise its well-being. Often, the cause of pleural pathology can be determined only after removal of pleural fluid or air, and follow-up radiographs should therefore always be made after thoracocentesis. Positive contrast peritoneography and gastrointestinal contrast studies may be of value in making a diagnosis when diaphragmatic rupture is present. Lymphangiography is a useful technique for planning thoracic duct ligation in animals with chylothorax: injection of contrast medium into mesenteric lymph nodes or lymphatics is described but requires laparotomy or laparoscopy; a less invasive technique is to inject contrast medium percutaneously into the popliteal lymph nodes, if necessary under ultrasound guidance. Pleurography is now rarely performed. If diagnostic ultrasound is available, it should be performed before draining any pleural fluid (see 8.5).
8.2. INCREASED RADIOLUCENCY OF THE PLEURAL CAVITY
Increased radiolucency results from air within the pleural cavity. The adjacent lung will collapse to a variable degree, making lung edges visible because free air is more radiolucent than the air–interstitium content of the lung. The lungs show an increased radiopacity due to reduced air content.
1. Artefactual increased radiolucency of the pleural cavity – on careful examination, often requiring a hot light, pulmonary blood vessels will be seen in the area suspected of containing free air.
a. Overexposure, overdevelopment or fogging of the film.
b. Überschwinger (halo artefact, rebound effect) on digital radiographs (see Appendix).
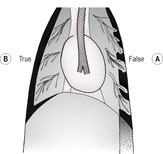
c. Lateral to superimposed axillary folds on the DV view, especially in deep-chested dogs; these can usually be followed outside the thoracic cavity (so-called false pneumothorax –Fig. 8.2A).
d. Overinflation of the lungs (see 6.25).
e. Deep inspiration.
f. Hypovolaemia and pulmonary undercirculation.
g. Subcutaneous emphysema.
h. Lobar emphysema.
2. Pneumothorax – variable degrees of retraction of the lungs from the thoracic wall and spine, with surrounding gas lucency and corresponding increase in lung opacity (Fig. 8.2B); the cardiac apex will be separated from the sternum on lateral recumbent radiographs, as support from the dependent lung is lost and the heart falls under gravity into the dependent hemithorax (differentiate from microcardia – see 7.4 and from pulmonary emphysema – see 6.25.6; in both of these, the heart apex may also be separated from the sternum for different reasons). Expiratory radiographs and left lateral recumbent views are more sensitive for the detection of small amounts of free air; alternatively, a standing lateral radiograph or a VD radiograph using a horizontal beam with the patient in lateral recumbency can be used – free air will collect beneath the uppermost part of the spine or rib cage, respectively. Pneumothorax is usually bilateral and symmetrical; focal areas of gas accumulation suggest underlying lung lobe pathology. Flattening and caudal displacement of the diaphragm together with outwardly bulging intercostal soft tissue suggest tension pneumothorax and prompt treatment is required.
a. Trauma with perforation of:
– Lung and visceral pleura.
– Thoracic wall and parietal pleura.
b. Spontaneous pneumothorax – tends to be recurring and may be life-threatening; especially large or deep-chested dogs. Classified as primary (rupture of a subpleural bulla or bleb without underlying lung pathology) or secondary (pre-existing lung disease). The underlying cause is usually very difficult to see radiographically and may be diagnosed only during surgery or post-mortem examination. CT is likely to be more rewarding.
– Rupture of congenital or acquired bulla (between layers of visceral pleura) or bleb (beneath the internal layer of visceral pleura), most often in the apical area.
– Bullous emphysema – the commonest underlying cause.
– Rupture of pulmonary cyst.
– Associated with small airway disease in cats.
– Bacterial pneumonia.
– Lung abscess.
– Neoplasia.
– Pleural adhesions.
– Parasitic lesions (Paragonimus*, Oslerus* and Dirofilaria*).
– Migrating pulmonary foreign body (e.g. grass awn).
c. Perforations of:
– Oesophagus.
– Trachea.
– Bronchi.
– Cavitary mass.
d. Iatrogenic.
– Lung aspirates.
– Thoracotomy.
– Thoracocentesis.
– Neck surgery.
– Vigorous cardiac massage.
– Artificial ventilation with a respirator.
f. Infection with gas-producing organisms.
3. Diaphragmatic rupture – displaced, gas-filled gastrointestinal tract may result in localized areas of increased radiolucency in the pleural cavity (Fig. 8.3). The wall of the stomach or intestine is usually clearly seen because of enteric gas inside and pulmonic air outside the wall, and mineralized fragments in ingesta may also be visible.
a. Large radiolucency on the left side of the thorax – herniated and dilated stomach.
b. Small tubular radiolucencies – herniated small intestine; may enlarge with obstruction or incarceration (also seen with pericardioperitoneal hernia, in which intestine lies within the pericardial sac rather than the pleural cavity).
4. Hydropneumothorax – VD radiographs made with a horizontal beam and the patient in lateral recumbency may be required – usually more fluid than air is present.
a. Pyopneumothorax – most common.
– Ruptured pulmonary abscess with bronchopleural fistula.
– Perforating oesophageal foreign body or Spirocerca lupi* granuloma.
b. Haemopneumothorax.
– Following trauma.
– Iatrogenic following thoracocentesis.
8.3. INCREASED RADIOPACITY OF THE PLEURAL CAVITY
Lung edges are displaced from the thoracic wall and become visible due to the difference between soft tissue opacity peripherally and air-filled lung centrally (Fig. 8.4A–C). Increased opacity of the pleural cavity must be distinguished from artefactual or genuine increases in lung opacity (see 6.13, 6.14 and 6.22). With severe effusions the heart is obscured, especially on lateral views, due to border effacement. However, in some animals a pericardial fat line is still visible, which suggests the location and size of the heart and may help in deciding whether cardiomegaly is present.
1. Fat opacity – in obese patients, a large sternal fat pad and a thinner layer of subpleural fat may be seen mimicking effusion. Fat may also accumulate in the pericardial sac. On careful examination, the fat will be seen to be less radiopaque than the adjacent cardiac and diaphragmatic silhouettes and no fissure lines will be seen.
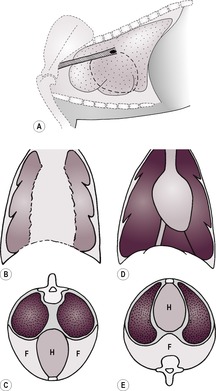
2. Pleural effusion – pleural fluid creates interlobar fissure lines (see 8.6.2 and Fig. 8.4A), scalloping of the ventral lung margins, retraction of the lungs from the thoracic wall and border effacement of the heart and diaphragm. On DV views fissure lines, and border effacement of the heart and diaphragm are seen (Fig. 8.4B, C)whereas on VD views the lung edges are rounded at the costophrenic angle and the heart may still be visible, as the fluid drains into the dorsal (dependent) part of the thorax (Fig. 8.4D and E). Small amounts of fluid are best seen on expiratory radiographs when the thoracic volume is smaller, or on horizontal beam VD views with the affected side down and the beam centred on the lower rib cage. Increasing volumes of fluid result in greater border effacement of the heart and diaphragm, with pulmonary opacity approaching that of the fluid as the lungs collapse and contain less air. Fluid may be free and move with gravity or may be encapsulated or trapped. Fluid collecting around a single lung lobe suggests underlying lobar pathology. All fluids have the same radiographic opacity, and thoracocentesis is required to establish the type of fluid present; repeat radiographic examinations should be made after draining the fluid, to evaluate degree of success of fluid removal and to evaluate the lungs, mediastinum and thoracic wall more completely. The presence of simultaneous pleural and peritoneal effusions carries a poorer prognosis.
a. Artefactual increased radiopacity of the pleural cavity.
– In obese dogs and cats, due to fat; see above.
– In normal cats, the caudodorsal lung margins are separated from the thoracic spine on the lateral view by the intrathoracic psoas muscle.
– In chondrodystrophic breeds, the costochondral junctions are indented medially, which may mimic pleural effusion on the DV or VD radiograph.
b. Transudate or modified transudate; likely to be bilateral.
– Heart failure (especially in cats).
– Neoplasia, especially lymphoma and mesothelioma; also may occur in association with ovarian tumours in dogs.
– Liver lobe incarcerated in a diaphragmatic rupture.
– Idiopathic effusion.
– Sterile foreign body.
– Pneumonia.
– Hypoalbuminaemia (nephrotic syndrome, protein-losing enteropathy, chronic liver failure).
– Lung lobe torsion.
– Glomerulonephritis.
– Pulmonary thromboembolism – mild.
– Subarachnoid–pleural fistula (rare).
– Urothorax (urine) secondary to kidney displaced through a ruptured diaphragm.
– Cats – hyperthyroidism with or without heart failure.
– Cats – secondary to perinephric pseudocyst.
c. Exudate; more likely to be unilateral or asymmetrical, as often inflammatory.
– Pyothorax.
– Pulmonary abscess.
– Foreign body (e.g. migrating grass awn).
– Nocardiosis*.
– Actinomycosis* – often in combination with mediastinal or pulmonary masses ± rib osteomyelitis.
– Tuberculosis.
– Pneumonia.
– Fungal effusions.
– Autoimmune disorders (e.g. systemic lupus erythematosus and rheumatoid arthritis) – usually small volumes.
– Neoplasia; mesothelioma most likely (rib and sternal lesions may also be seen).
– Bilothorax – trauma (e.g. gunshot) or secondary to cholecystitis, although mechanism for the latter unknown; secondary to biloperitoneum in presence of ruptured diaphragm.
– Cats – feline infectious peritonitis (FIP).
d. Chyle – conditions that cause increased right-sided venous pressure or obstruction of flow of lymph into the venous system; provokes an exudative response too.
– Right heart failure, especially cats (may result in constrictive pleuritis).
– Cardiomyopathy.
– Congenital heart disease.
– Pericardial effusion.
– Trauma, rupturing thoracic duct.
– Cranial mediastinal mass.
– Neoplasia.
– Lung lobe torsion.
– Thrombosis.
– Fibrosis.
– Congenital (e.g. abnormal termination of the thoracic duct).
f. Pleural fluid may also arise secondary to abdominal effusion, crossing the diaphragm via lymphatics, for example.
– Transudate secondary to liver disease.
– Exudate secondary to pancreatitis.
8.4. PLEURAL AND EXTRAPLEURAL NODULES AND MASSES
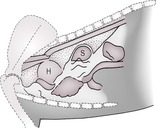
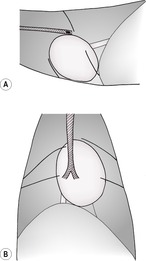
2. Extrapleural masses – these bulge into the pleural cavity from the parietal side of the chest wall, creating an extrapleural sign characterized by a well-demarcated, convex contour with tapering cranial and caudal edges (Fig. 8.5) Such lesions have a tendency to grow inwards rather than outwards and may widen the adjacent intercostal spaces and involve the ribs. They do not move with respiratory motion of the lung on fluoroscopy or ultrasonography. There is no (or minimal) pleural effusion unless the disease process has extended into the pleural cavity. Special oblique radiographs may be required to skyline the pathology.
a. Rib tumours (see 8.22.5).
b. Inflammatory conditions.
– Osteomyelitis of the osseous thoracic wall structures.
– Abscess.
– Granuloma.
– Foreign body reaction.
c. Soft tissue tumours.
– Lipoma – fat radiopacity usually obvious.
– Haemangiosarcoma.
– Fibrosarcoma.
– Rhabdomyosarcoma.
e. Haematoma – as result of trauma and associated rib fractures.
3. Small diaphragmatic ruptures, hernias and eventration – sometimes incidental findings (see 8.26.1).
4. Pleural tumours – visible only after pleural drainage and if large enough.
a. Mesothelioma – usually multiple or diffuse.
b. Metastatic carcinomatosis – also diffuse.
5. Pleural abscess or granuloma (e.g. secondary to foreign body).
6. Encapsulated or loculated pleural fluid – does not move with gravity.
7. Pleural fluid collecting around a diseased lung lobe.
8. Fibrin remnants after pleural drainage.
8.5. ULTRASONOGRAPHY OF PLEURAL AND EXTRAPLEURAL LESIONS
Ultrasonography complements radiography in the investigation of pleural disease, especially when free fluid is present, and also allows ultrasound-guided aspiration of pockets of fluid or small lesions. Intercostal, parasternal, transhepatic and thoracic inlet approaches may be used. It may be helpful to scan the patient from different approaches, including the ventral aspect in which the effect of gravity may cause fluid to pool and create a useful acoustic window.
1. Pleural effusion – the ultrasonographic appearance of pleural fluid is variable but is usually anechoic to hypoechoic. Fluid surrounds and separates the lung lobes from each other and the thoracic wall, and acts as a useful acoustic window to examine other parts of the thorax. Many echoes within the fluid usually signify the presence of clumps of cells, debris and/or gas bubbles, although thoracocentesis is required to determine the nature of the fluid. It also facilitates imaging of intrathoracic structures that are not usually seen, such as the great vessels in the cranial mediastinum. The identification of echogenic tags and deposits on pleural surfaces is suggestive of the presence of an exudate, blood or chyle or a diffuse tumour such as mesothelioma. Mesothelioma may also produce cauliflower-like masses, especially in the dorsal part of the thoracic cavity. Inflammatory effusions may result in a septated appearance. For possible causes of pleural effusion, see 8.3.2.
Severe pleural effusion may cause right atrial collapse, mimicking cardiac tamponade secondary to pericardial effusion.
2. Hypoechoic or anechoic, well-circumscribed areas.
a. Encapsulated or trapped fluid.
b. Pleural abscess.
c. Haematoma.
d. Sternal lymphadenopathy.
e. Soft tissue tumour of homogeneous cellularity and with little haemorrhage or necrosis.
f. Ectopic liver or a small portion of liver prolapsed through a diaphragmatic tear.
3. Heterogeneous area.
a. Rib or sternal tumour.
b. Soft tissue tumour of heterogeneous cellularity and/or fibrosis, calcification, necrosis or haemorrhage.
c. Inflammatory conditions.
– Abscess.
– Granuloma.
– Foreign body reaction.
4. Viscera within the thorax – the identification of abdominal viscera (e.g. liver, spleen, gastrointestinal tract) within the thoracic cavity is a more certain ultrasonographic indicator of diaphragmatic rupture than identification of the diaphragmatic defect. Variable quantities of thoracic fluid may also be seen.
a. Artefactual due to mirror image artefact, giving the impression of liver tissue within the thorax when scanning transhepatically (see Appendix).
b. Viscera not contained within the pericardium – traumatic diaphragmatic rupture.
c. Viscera apparently contained within the pericardium – congenital peritoneopericardial diaphragmatic hernia.
8.6. PLEURAL THICKENING: INCREASED VISIBILITY OF LUNG LOBE EDGES
Lungs normally extend to the periphery of the thoracic cavity, and individual lobar edges are not seen except in two locations:
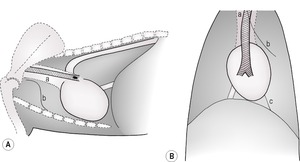
• In the cranioventral thorax, where the mediastinum runs obliquely and outlines the cranial segment of the left cranial lobe on a lateral radiograph (see 8.7 and Fig. 8.7A).
• Along the ventral margins of the lungs, which may appear scalloped in some dogs on the lateral radiograph due to intrathoracic fat.
1. Retracted lung borders making the edges visible.
a. Artefactual.
– Axillary skin folds or skin folds created by a foam wedge placed under the sternum – the line extends beyond the thorax, and pulmonary vasculature is visible peripheral to the line.
– Inwardly displaced costochondral junctions in chondrodystrophic breeds, especially the Dachshund and Bassett Hound, creating a false impression of pleural fluid on the DV view.
c. Pneumothorax.
d. Pleural effusion.
e. Constrictive pleuritis secondary to pyo- or chylothorax (cortication).
f. Atelectasis.
2. Fissure lines – thin, radiopaque lines along the lobar borders (Fig. 8.6A and B).
a. Artefactual.
– Thin, mineralized costal cartilages (on the DV view these tend to be concave cranially, whereas fissure lines are concave caudally).
– Scapular spine or edges.
b. Incidental – a fine fissure line is occasionally seen over the heart on left lateral radiographs of larger dogs.
c. Mild pleural effusion – fissure lines are wider peripherally than centrally.
d. Fibrinous pleuritis (cortication) secondary to pyo- or chylothorax – especially in cats. Rounded lung borders outlined by fine, radiopaque lines are seen as the lungs fail to re-expand fully after thoracocentesis.
e. Pleural fibrosis or scarring – fine lines of uniform width.
– Old age and healed disease.
– Fungal disease (e.g. coccidioidomycosis* and nocardiosis*).
– Parasitic disease (e.g. Filaroides hirthi and F. milksi*).
f. Pleural oedema in left heart failure.
g. Dry pleuritis (e.g. migrating intrathoracic grass awns in working dogs).
3. Peripheral lobar consolidation or collapse highlighting interfaces with adjacent air-filled lobes.
MEDIASTINUM
8.7. ANATOMY AND RADIOGRAPHY OF THE MEDIASTINUM
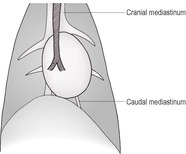
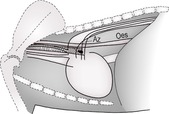
The mediastinum is the space between the two pleural sacs and consists of two layers of mediastinal pleura, which separate the thorax into two pleural cavities. It accommodates a large number of structures, including the heart, large blood vessels, trachea, oesophagus and lymph nodes, and lies roughly in the midline (Figs 8.1 and 8.7A and B). Anatomically, it may be divided into cranial (precardiac), middle (cardiac) and caudal (post-cardiac) parts. The cranial part lies between the thoracic inlet and the heart and contains major blood vessels, trachea, oesophagus, lymph nodes, vagus nerve and thymus gland. The middle part contains the heart and major vessels, the terminal trachea and tracheobronchial lymph nodes. The caudal part lies between the heart and the diaphragm and contains the descending aorta, caudal vena cava (CdVC) and oesophagus. The mediastinum communicates cranially with fascial planes of the neck and caudally with the retroperitoneal space via the aortic hiatus. Cranial to the heart, the large dorsal and central soft tissue radiopacity is formed from the cranial thoracic blood vessels, oesophagus, trachea and lymph nodes. On DV or VD radiographs, the width of the cranial mediastinum in dogs should not normally exceed twice the width of the vertebral bodies. Ventrally, the cranial mediastinum forms a thin soft tissue fold running obliquely from craniodorsal to caudoventral on the lateral view. On DV or VD radiographs, it extends from craniomedial in a caudolateral direction to the left side, separating the right and left cranial lung lobes. This fold contains the sternal lymph node ventrally, and the thymus in young animals. Caudally, the ventral mediastinum is seen on DV or VD radiographs as a fold displaced into the left hemithorax by the accessory lung lobe. In cats, the width of the craniodorsal mediastinum is less than the width of the superimposed thoracic vertebrae on the DV or VD view and the cranioventral fold is difficult to see.
Apart from the heart, aorta, CdVC and trachea, which are surrounded by air, mediastinal structures cannot be identified separately, as they have similar soft tissue opacity and are in contact with each other. Surprisingly, the caudal oesophagus is rarely visible. The DV or VD view is usually more informative than the lateral for the investigation of mediastinal disease, with the exception of pneumomediastinum, although both views should be obtained. As for pleural disease, it is important to obtain orthogonal radiographs, and positional radiography as described in Section 8.1 may also be helpful when mediastinal and/or pleural fluid are present.
8.8. MEDIASTINAL SHIFT
Mediastinal shift is diagnosed by evaluating the position of the heart, trachea, main stem bronchi, aortic arch and vena cava on well-positioned DV or VD views.
1. Artefactual.
a. Oblique DV or VD views.
2. Uneven inflation of the two hemithoraces due to unilateral pathology.
Mediastinal movement towards the affected hemithorax
a. Unilateral atelectasis of the lung.
– General anaesthesia and lateral recumbency (may occur within a few minutes of induction, especially in large dogs).
– Prolonged lateral recumbency with severe illness.
– Faulty intubation – endotracheal tube in one bronchus.
– Mass or foreign body obstructing a bronchus.
– Cats – feline bronchial asthma with lobar bronchus obstruction.
b. Lung lobe torsion.
c. Lobectomy.
d. Lobar aplasia or hypoplasia.
e. Radiation-induced fibrosis and atelectasis.
f. Adhesions.
g. Unilateral phrenic nerve paralysis.
Mediastinal movement away from the affected hemithorax
3. Chronic pleural disease with adhesions.
8.9. VARIATIONS IN MEDIASTINAL RADIOPACITY
Most mediastinal changes are of soft tissue opacity, but the mediastinum may also be less radiopaque due to the presence of fat or air, or more radiopaque due to mineralization.
Reduced mediastinal radiopacity due to air: pneumomediastinum
Generalized pneumomediastinum with dissecting radiolucencies results in increased visibility of mediastinal structures such as blood vessels, tracheal walls and oesophagus (Fig. 8.8). Air may extend into the fascial planes of the neck and progress to subcutaneous emphysema, into the retroperitoneum and into the pericardium (rare). Occasionally, localized pneumomediastinum is seen as pockets of mediastinal air. An air-filled megaoesophagus will also produce mediastinal widening of air lucency and will increase the visibility of the dorsal tracheal wall (see 8.17) and should not be mistaken for pneumomediastinum. Pneumomediastinum may lead to pneumothorax, but the reverse does not occur. Compression of venous structures may cause circulatory collapse.
1. Artefact – gas-filled megaoesophagus superimposed over cranial mediastinal vessels.
2. Iatrogenic pneumomediastinum.
a. Post transtracheal aspiration.
b. Post lung aspirate.
c. Overinflation of the lungs during positive pressure ventilation.
d. Post endoscopy.
e. Trauma from endotracheal intubation.
f. In cats, following overinflation of the cuff of an endotracheal tube.
3. Extension of air from the neck.
a. Soft tissue trauma of the head or neck, with an open wound.
b. Cervical tracheal perforation.
c. Cervical oesophageal perforation.
d. Pharyngeal perforation.
e. Jugular venepuncture.
f. Soft tissue infection with gas formation.
4. Extension of air from the bronchi or lungs; air dissecting along perivascular or peribronchial adventitia.
a. Rupture of the bronchi or lungs.
– Rupture of pulmonary bulla, bleb or cyst.
– Bronchial parasitism.
– Compressive trauma.
– Lung lobe torsion.
– Lobar emphysema.
b. Spontaneous pneumomediastinum – racing Greyhounds.
5. Tracheal perforation within the thorax.
6. Oesophageal perforation within the thorax.
8. Emphysematous mediastinitis.
9. Extension from pneumoretroperitoneum.
Reduced mediastinal radiopacity: fat
10. Obesity – especially in chondrodystrophic dogs.
Increased mediastinal radiopacity, greater than soft tissue
11. Iatrogenic.
a. Intravenous or intra-arterial catheter.
b. Endotracheal tube.
c. Feeding tube.
d. Oesophageal stethoscope.
12. Food material in distended oesophagus – see 8.18.
13. Mineralization.
a. Mineralized oesophageal foreign bodies (see 8.20).
b. Mineralized fragments of ingesta accumulating in a dilated oesophagus (e.g. secondary to vascular ring anomaly).
c. Neoplastic mass.
– Osteosarcoma transformation of Spirocerca lupi* granuloma.
– Thymic tumour.
– Lymphoma.
– Metastatic mediastinal tumour.
d. Chronic infectious lymph node involvement (see 8.12) (e.g. histoplasmosis* or tuberculosis).
e. Cardiovascular mineralization – aorta, coronary vessels and heart valves (see 6.27.7).
14. Metal.
a. Bullets and other metallic foreign bodies.
b. Contrast media.
– Leaking from a perforated oesophagus.
– Positive contrast peritoneography with ruptured diaphragm.
– Previously aspirated barium accumulating in hilar lymph nodes.
8.10. MEDIASTINAL WIDENING
Generalized mediastinal widening may be caused by accumulation of fat or fluid. Mediastinal fluid may result in reverse fissure lines as fluid dissects into the interlobar fissures from the hilar region. The reverse fissure lines are wider centrally and narrower peripherally (Fig. 8.9) and should not be confused with atelectatic lung lobes, especially a collapsed right middle lobe, which may appear small and triangular (see 6.17.3 and Fig. 6.10). Reverse fissures may also arise with pleural effusions but in these animals are likely to be obscured by the fact that the fluid is distributed over a larger area. Localized or walled-off accumulations of fluid mimic mediastinal masses (see 8.11). Mediastinal fluid may extend into the pleural space, but the reverse does not occur.




1. Incidental mediastinal widening.
a. A widened cranial mediastinum is routinely seen on DV or VD radiographs of Bulldogs. The trachea is in its normal position, slightly to the right of the midline.
b. In obese patients, especially in small and miniature breeds, large fat deposits result in a widened, smoothly marginated cranial mediastinum. The trachea is in its normal position, slightly to the right of the midline.
c. Thymic ‘sail’ on DV or VD view in young animals, between the right and left cranial lung lobes.
2. Mediastinal masses (see 8.11).
3. Oesophageal dilation (see 8.17).
4. Haemorrhage (for list of causes, see 8.3.2).
5. Mediastinitis or mediastinal abscess secondary to:
a. oesophageal or tracheal perforation
c. Spirocerca lupi*
d. cats – mediastinal feline infectious peritonitis.
6. Oedema or transudate (often with pleural fluid too).
a. Acute systemic disease.
b. Trauma.
c. Hypoproteinaemia.
d. Right heart failure.
e. Neoplasia, especially with cranial mediastinal masses in cats.
7. Chylomediastinum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree