abdominal wall, peritoneal and retroperitoneal cavities, parenchymal organs
CHAPTER CONTENTS
Retroperitoneal space236
9.9 Enlargement of the retroperitoneal space236
9.10 Increased radiopacity of the retroperitoneal space and/or loss of visualization of the retroperitoneal structures237
9.11 Decreased radiopacity of the retroperitoneal space238
9.12 Ultrasonography of the retroperitoneal space238
9.13 Ultrasonography of the lymph nodes in the retroperitoneal space238
9.14 Ultrasonography of the abdominal aorta and caudal vena cava238
Liver239
9.15 Displacement of the liver239
9.16 Variations in liver size239
9.17 Variations in liver shape241
9.18 Variations in liver radiopacity242
9.19 Hepatic contrast studies242
9.20 Ultrasonographic technique for the liver245
9.21 Normal ultrasonographic appearance of the liver245
9.22 Ultrasonographic abnormalities of the hepatic parenchyma245
9.23 Ultrasonographic abnormalities of the biliary tract247
9.24 Ultrasonographic abnormalities of the hepatic vascular system247
Spleen248
9.25 Absence of the splenic shadow248
9.26 Variations in location of the spleen248
9.27 Variations in splenic size and shape249
9.28 Variations in splenic radiopacity250
9.29 Ultrasonographic technique for the spleen250
9.30 Normal ultrasonographic appearance of the spleen250
9.31 Ultrasonographic abnormalities of the spleen250
9.1. RADIOGRAPHIC TECHNIQUE FOR THE ABDOMEN AND EFFECT OF POSITIONING
Radiography should be performed when possible as an elective procedure to ensure an empty gastrointestinal tract, as a food-filled stomach or faeces-filled colon may severely hamper interpretation. Lateral and ventrodorsal (VD) positions are the standard. The choice between right lateral recumbent (RLR) and left lateral recumbent (LLR) is a personal one, but whichever is chosen it should be used consistently to familiarize the user with its normal appearance. The differences between the two lateral views are described in Table 9.1. Collimation should include the whole diaphragm and the pelvic inlet. In animals with urinary obstruction, the radiograph should extend caudally to include the whole perineal and urethral area; in large dogs, this may require two separate radiographs. Exposure should be made on expiration to avoid motion blur. Left lateral decubitus views can be used to detect free abdominal gas (see 9.7).
| Anatomical Area | Right Lateral Recumbency | Left Lateral Recumbency |
|---|---|---|
| Gas in stomach | Gas in the fundus and body; fluid gravitates to the dependent pylorus, where it may mimic a round soft tissue opacity, foreign body or mass | Gas in the pylorus |
| Gas in proximal duodenum | Not seen | Often seen |
| Kidneys | Less superimposition | More superimposition |
| Liver | Appears smaller than on left lateral recumbency | Appears larger than on right lateral recumbency |
| Spleen | Often seen | Rarely seen |
For digital radiography, an appropriate algorithm should be used to combine resolution and contrast. For conventional film radiography, a fast film–screen combination should be used to minimize motion blur, and a grid should be employed if the abdomen is > 10 cm thick or in smaller, obese dogs. A short-scale contrast technique (low kV, high mAs) will improve the naturally low contrast of the abdominal organs. Increased abdominal detail can be obtained by trying to maximize organ separation. This can be achieved by placing the dog in dorsal rather than sternal recumbency, positioning the pelvic limbs perpendicular to the spine and making the exposure on expiration.
Optimal evaluation of abdominal radiographs requires a systematic approach, which involves assessing radiographic technique, signalment (breed type, age, sex, body condition), extra-abdominal structures (superficial soft tissues, osseous structures, caudal thorax and diaphragm) and intra-abdominal structures, and then re-evaluating abnormalities and areas in the light of the history and clinical examination. Intra-abdominal evaluation may be done on a system basis: gastrointestinal, urinary tract, reproductive tract, parenchymatous organs (liver and spleen), endocrine organs (adrenals and pancreas), peritoneal cavity and retroperitoneal space, and lymphatic system (sublumbar lymph nodes). Alternatively, a quadrant approach can be used. For viewing abdominal radiographs, the convention is that lateral radiographs are examined with the diaphragm facing to the left, and VD radiographs with the diaphragm uppermost and the left side of the patient on the right side of the computer screen or light box. Abdominal fat in smaller dogs and in cats helps to outline abdominal structures, as the lower fat opacity contrasts well with the soft tissue opacity of the majority of abdominal organs. However, in large obese dogs this advantage is lost, as the large amounts of fat result in excessive amounts of scatter, which degrades the image despite the use of a grid.
If an organ that is normally visible is not seen despite good-quality radiographs, pathology is implied. Similarly, if an organ that is normally not seen becomes partly or completely visible, disease is also likely. Structures normally visible and not visible on abdominal radiograph are listed in Box 9.1. There are some radiographic anatomical differences between the dog and cat, and these are given in Table 9.2.
BOX 9.1
Normally visible
▪ Ventral liver lobes.
▪ Spleen on right lateral recumbent view.
▪ Stomach.
▪ Small intestines.
▪ Caecum.
▪ Colon.
▪ Kidneys.
▪ Bladder.
▪ Prostate (not young dogs).
▪ Peritoneal cavity.
▪ Retroperitoneal space.
Normally not visible
▪ Pancreas.
▪ Adrenals.
▪ Gall bladder.
▪ Ureters.
▪ Urethra.
▪ Ovaries.
▪ Non-gravid uterus.
▪ Lymph nodes.
▪ Blood vessels.
| Anatomical Area | Dog | Cat |
|---|---|---|
| Gastric and duodenal position on the ventrodorsal view | Lies transversely within the costal arch, and the descending duodenum is adjacent to the right body wall | Pylorus is in the midline with an acute angular incisure, and descending duodenum lies slightly to the right of the midline |
| Gastric layering | Not seen | May be seen due to fat deposition in wall |
| Kidneys | Whole left and caudal pole of right usually seen | Both kidneys seen; fat may be seen in the renal pelvis in obese cats |
| Bladder location | Bladder neck prepubic | Due to long urethra, lies more cranially in the abdomen |
| Spleen | Readily seen on right lateral recumbent views | Not usually seen in the ventral abdomen on lateral views |
| Caecum | Seen in the right mid-abdomen as gas-filled structure | Not seen |
| Adrenals | Not seen | Mineralized adrenals are occasionally seen as an incidental finding |
| Abdominal fat | May be present | Often large amounts, which provides good abdominal contrast |
9.2. ULTRASONOGRAPHIC TECHNIQUE FOR THE ABDOMEN
Curvilinear or sector transducers allow optimal access to intra-abdominal structures, although linear array transducers may also be used. As high a frequency as possible should be selected while still achieving adequate tissue penetration (e.g. 7.5MHz for cats or small dogs and 5MHz for medium or large dogs).
The chosen acoustic window should be carefully prepared by clipping hair from the area, cleaning the skin with surgical spirit to remove dirt and grease, and applying liberal quantities of acoustic gel. In general, it is preferable to do a complete abdominal examination in each patient rather than restricting the examination to an individual organ system. It is essential to follow a systematic approach to the examination to ensure that no organ is missed. The precise order of the examination is not important, but it can be helpful to image the easier organs first to allow the patient to settle into the procedure. The patient may be in lateral or dorsal recumbency depending on personal preference. The position of the animal can be altered if necessary to make use of the effects of gravity on the distribution of intestine filled with large amounts of gas, or evaluating the mobility of suspect hyperechoic structures within fluid-filled structures.
Ultrasound-guided fine needle aspiration using a 22-gauge needle or spinal needle for deeper structures can be readily performed. With increasing expertise, the needle can be guided into small pathological areas. Alternatively, a tissue core biopsy can be performed under ultrasound guidance after ensuring that clotting factors are normal.
The selected acoustic window should be prepared aseptically and the transducer carefully cleaned. A sterile transducer sleeve can be used to cover the transducer if desired. The tissue or organ of interest should be imaged, and the position of the transducer adjusted until the region to be sampled is clearly seen and there is space to introduce the needle safely at an appropriate angle adjacent to the transducer.
In order to aspirate fluid, the needle is introduced until the tip is seen clearly within the fluid. If necessary, the transducer may be rocked gently from side to side to identify the needle. When positioning is confirmed, fluid is aspirated then the needle is withdrawn.
For fine needle aspiration of a solid organ, the needle is introduced as described above into the tissue of interest. When the needle tip is in position, a gentle in and out movement of the needle is made, and it is then withdrawn. If this does not yield a sample, the procedure may be repeated, applying gentle suction when the needle is in position.
For a tissue core biopsy, the needle is introduced as above and samples of tissue are collected from both the centre and the periphery of the lesion.
ABDOMINAL WALL
The abdominal wall is formed by the diaphragm and rib cage cranially, abdominal muscles ventrally and laterally, sublumbar muscles dorsally and peritoneum caudally.
9.3. VARIATIONS IN SHAPE OF THE ABDOMINAL WALL
1. Generalized distension of the abdominal wall.
a. Obesity – abdominal viscera well outlined by fat; large falciform fat pad.
b. Loss of muscle tone, resulting in sagging of abdominal structures.
– Old age.
– Cushing’s syndrome (hyperadrenocorticism) – naturally occurring or iatrogenic.
c. Large abdominal mass, especially splenic.
e. Small intestinal distension (see 10.21.3 and Fig. 10.10, e.g. low obstruction).
g. Uterine distension in female animals.
– Mid- to late-term pregnancy.
– Large pyometra.
– Large mucometra, haemometra or hydrometra.
h. Severe peritoneal effusion.
– Right heart failure.
– Liver disease.
– Nephrotic syndrome.
– Other causes of hypoproteinaemia.
– Obstruction of the caudal vena cava (CdVC).
– Ruptured urinary bladder.
– Intra-abdominal haemorrhage.
– Cats – feline infectious peritonitis (FIP).
i. Severe pneumoperitoneum.
2. Focal distension of the abdominal wall.
a. Umbilical hernia.
c. Traumatic rupture of abdominal or paracostal muscles.
d. Surgical wound breakdown.
In (a)–(d), viscera may be contained within the focal distension (a contrast study or ultrasonography may be helpful if this is unclear on plain radiographs).
f. Abdominal wall haematoma.
g. Abdominal wall seroma.
h. Abdominal wall neoplasm.
i. Lipoma – fat opacity.
3. Inward displacement of the abdominal wall.
a. Emaciation.
b. Diaphragmatic rupture with herniation of abdominal viscera into the thoracic cavity.
c. Peritoneopericardial diaphragmatic hernia, with movement of abdominal viscera into the pericardial sac.
d. Severe inspiratory dyspnoea.
4. Thickening of the abdominal wall.
a. After laparotomy.
b. Trauma – contusion, haemorrhage or rupture.
9.4. VARIATIONS IN RADIOPACITY OF THE ABDOMINAL WALL
In a well-nourished adult animal, fat interspersed between the fascial planes allows visualization of the various muscle layers.
1. Increased soft tissue radiopacity and loss of distinction of muscle layers of the abdominal wall.
a. Trauma with oedema or haemorrhage of the soft tissues.
b. Abdominal wall neoplasia.
c. Large volumes of fluid administered subcutaneously.
d. Cellulitis.
e. Healing laparotomy.
2. Mineralized radiopacity of the abdominal wall.
a. Overlying wire skin sutures or staples.
b. Overlying wet or dirty hair coat or ultrasound gel mimicking mineralized radiopacity.
c. Calcinosis cutis associated with hyperadrenocorticism (Cushing’s disease).
d. Mineralization of superficial skin masses.
e. Foreign material (e.g. ballistics, dirt in the hair coat).
f. Ointments or lotions containing radiopaque compounds (e.g. iodine, zinc) applied to the skin surface.
3. Decreased radiopacity of the abdominal wall.
a. Fat – lipoma or liposarcoma.
b. Gas.
– Local skin lacerations or defects.
– Subcutaneous emphysema extending from a wound elsewhere.
– Gas dissecting along fascial planes from a pneumomediastinum or pneumoretroperitoneum.
– Recent surgical incision.
– Gas within a hernia that contains small intestine.
9.5. ULTRASONOGRAPHY OF THE ABDOMINAL WALL
Interpretation is similar to that of ultrasonography of the soft tissues of the thoracic wall (see 8.24).
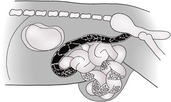
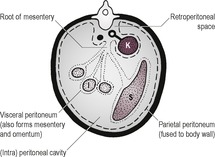
PERITONEAL CAVITY
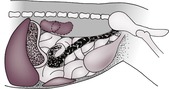
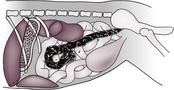
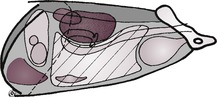
The abdominal cavity is lined by peritoneum, and the areas between the major organs and the intestine are known as the peritoneal cavity (Fig. 9.2). In the normal adult animal, serosal detail of abdominal viscera is demonstrated by intra-abdominal fat.
9.6. INCREASED RADIOPACITY OF THE PERITONEAL CAVITY AND/OR LOSS OF VISUALIZATION OF ABDOMINAL ORGANS
All causes of increased intra-abdominal radiopacity result in loss of serosal detail by obscuring intra-abdominal fat, which normally provides contrast with soft tissues. A diffuse and homogeneous increase in intra-abdominal opacity and loss of serosal detail is sometimes referred to as a ground glass appearance and is usually due to free abdominal fluid. Increase in opacity may also be patchy or mottled.
1. Generalized and homogeneous increase in radiopacity of the peritoneal cavity.
a. Normal animal – suboptimal radiograph.
– Underexposure.
– Underdevelopment.
– kVp setting too high, leading to reduced contrast.
– Scattered radiation if no grid has been used with a large abdomen.
b. Diffusely wet hair coat.
c. Normal puppy or kitten – lack of abdominal fat due to young age.
d. Emaciation and lack of abdominal fat – abdominal wall tucked inwards.
e. Peritoneal effusion – often abdominal distension too.
– Ascites (hydroperitoneum; Fig. 9.3): right heart failure, liver disease, portal hypertension, hypoproteinaemia, obstruction of the CdVC (see 9.16.1), neoplasia (specific organ, mesothelioma or carcinomatosis), other causes.
– Haemoperitoneum: ruptured abdominal tumour, particularly splenic haemangiosarcoma (especially German Shepherd dogs); coagulopathy (warfarin poisoning, thrombocytopenia, disseminated intravascular coagulation, congenital bleeding disorders, angiostrongylosis*); trauma, ruptured vascular anomaly, spontaneous liver rupture with amyloidosis (especially oriental cats).
– Uroabdomen – ruptured urinary bladder or urethra.
– Bile peritonitis – ruptured gall bladder or bile duct.
– Chylous effusion – may be due to neoplasia or hepatic portal vein obstruction.
– Cats – feline infectious peritonitis.
f. After peritoneal dialysis or other intraperitoneal fluid administration.
2. Generalized but heterogeneous increase in radiopacity of the peritoneal cavity.
a. Artefactual due to overlying wet or dirty hair coat.
b. Peritonitis.
– Recent laparotomy.
– Intestinal rupture – dilated intestinal loops and free gas in the peritoneal cavity are also likely; these findings warrant immediate surgical exploration.
– Trauma (e.g. penetrating wounds).
– Migrating gastrointestinal foreign body (e.g. wooden skewer).
– Pancreatitis.
– Bile or urine peritonitis.
c. Small peritoneal effusion – see above for list of possible causes (ultrasonography is more sensitive than radiography for the detection of small effusions).
d. Peritoneal carcinomatosis (usually metastatic) – due to neoplastic nodules and free fluid.
e. Cats – steatitis – large amounts of intra-abdominal fat of mottled, increased opacity.
– Vitamin E deficiency.
– Fish diet.
3. Localized increase in radiopacity of the peritoneal cavity – often heterogeneous.
a. Artefact – superimposed wet or dirty hair; ultrasound gel on coat; lotions or ointments containing radiopaque compounds.
b. Abdominal lymphadenopathy, usually neoplastic.
c. Localized abdominal trauma.
d. Intestinal perforation walled off by mesentery.
e. Pancreatitis – right cranial quadrant of abdomen.
g. Peritoneal spread from adjacent neoplastic mass.
h. Walled-off abscess.
i. Prostatitis.
j. Retained surgical swab.
4. Mineralized opacity in the peritoneal cavity – commoner causes (see 9.41 for a more comprehensive list).
a. Mineralized ingesta in the bowel.
b. Mineralized foreign bodies in the bowel.
c. Foreign body free in the peritoneal cavity.
d. Urolithiasis.
e. Pregnancy.
f. Cholelithiasis.
g. Paraprostatic cyst; wall often mineralized, with an eggshell appearance.
h. Dystrophic or metastatic mineralization of soft tissues (see 12.2.2).
– Neoplasia.
– Chronic haematoma or abscess.
– Hyperparathyroidism.
– Associated with retained surgical swab.
– Nodular fat necrosis – usually old, obese cats.
i. Barium or iodinated contrast medium leaking from perforated gastrointestinal or urogenital tract.
9.7. DECREASED RADIOPACITY OF THE PERITONEAL CAVITY
1. Fat opacity – intra-abdominal lipoma; viscera will be displaced by the lipoma.
2. Gas opacity – a small volume of free gas is detected most accurately by taking a standing lateral radiograph or an LLR VD view with a horizontal beam. With both views, gas will rise to the highest part of the peritoneal cavity once the patient has maintained the position for a few minutes. With larger amounts of free gas, the liver and diaphragm will be separated, outlining the diaphragm, and there will be increased visibility of the serosal surface of intestines and other organs.
a. Iatrogenic.
– Post laparotomy.
– Post peritoneal dialysis.
– Post paracentesis (small amounts of gas).
– Overdistension of bladder during pneumocystography, especially in cats with feline idiopathic cystitis.
– Retained surgical swab – small gas foci.
– Air embolism – gas in blood vessels.
– Pneumocoeliography.
b. Perforated hollow viscus (trauma, neoplasia, ulceration) – increased radiopacity may also be present due to peritonitis or free fluid.
– Perforated stomach – large volume of gas causing abdominal distension and spontaneous pneumoperitoneum.
– Perforated small or large intestine.
– Ruptured bladder with pneumocystogram performed.
c. Leakage of gas from emphysematous organ (e.g. stomach, bladder, colon or uterus).
d. Leakage of gas through an intact, distended stomach wall (e.g. gastric dilation and volvulus, GDV).
e. Entry of air through the abdominal wall.
– Penetrating wound.
– Around an abdominal drain or feeding tube.
f. Infection with gas-producing organisms.
g. Gas in an abscess (may see fluid line with a horizontal beam).
h. Pneumothorax with a diaphragmatic rupture.
i. Post-mortem change in major vessels, occurring within 12h of death.
9.8. ULTRASONOGRAPHY OF THE PERITONEAL CAVITY
1. Peritoneal fluid – fluid (transudate or modified transudate) is generally anechoic but may contain echoes (exudate) depending on its cellular content or the presence of debris or small gas bubbles. Fluid surrounds and separates the abdominal organs, often enhancing visibility of these structures. In order to detect small quantities of fluid, the dependent portions of the abdomen should be examined. In particular, small accumulations of fluid may be found between the liver lobes, between the liver and the diaphragm, and around the urinary bladder. Ultrasonography is more sensitive than radiography for detecting small quantities of free fluid (Fig. 9.4). For differential diagnoses for the causes of peritoneal effusions, see 9.6.1.
2. Free gas – this results in a poor-quality image and multiple artefacts (shadowing and reverberation). The effect of free gas on image quality can be reduced by altering the position of the patient and imaging from the dependent aspect. Radiography is more sensitive than ultrasound for the detection of small quantities of free gas in the peritoneal cavity; the gas rises to the uppermost portion of the abdomen and in dorsal recumbency will be found in the sternal region. For differential diagnoses for the causes of free gas, see 9.7.2.
3. Peritoneal masses.
a. Carcinomatosis – parietal and visceral peritoneal masses and free fluid; may also see primary or metastatic masses in abdominal organs and enlarged abdominal lymph nodes.
b. Granuloma – may occur around a retained surgical sponge or swab, non-absorbable sutures or other foreign material; hypoechoic mass with central strong acoustic shadowing.
c. Abscess – wall of variable thickness and contents of variable echogenicity.
d. Cyst – thin-walled, mostly septated cysts are seen on the peritoneal surface of abdominal organs and omentum in peritoneal cestodiasis in dogs and cats; in the later stages, there may be abdominal fluid and peritoneal thickening.
RETROPERITONEAL SPACE
The retroperitoneal space (retroperitoneum) is the region of the abdomen ventral to the spine and dorsal to the intestines, lying outside the peritoneal cavity (see Fig. 9.2). The kidneys and prostate protrude into the peritoneal cavity from the retroperitoneal space and are partly covered by peritoneum. Retroperitoneal fat outlines the kidneys and ventral musculature of the spine. In fat animals, the deep circumflex arteries seen end on ventral to the caudal lumbar vertebrae may simulate the appearance of mineral opacities such as ureteric calculi. The aorta, CdVC and ureters are occasionally seen running through the retroperitoneal space in obese animals, but other retroperitoneal structures such as the adrenal glands and lymph nodes are not detectable when normal. The overall radiopacity of the retroperitoneal space should be similar to that of the peritoneal cavity.
9.9. ENLARGEMENT OF THE RETROPERITONEAL SPACE
1. Generalized retroperitoneal enlargement of fat opacity; normal visualization of the kidneys and sublumbar musculature; ventral displacement of the intestines.
a. Normal, obese animal; the kidneys are clearly seen, and occasionally blood vessels and ureters are visible.
b. Retroperitoneal lipoma.
2. Generalized retroperitoneal enlargement of soft tissue opacity; loss of visualization of the kidneys and sublumbar musculature; ventral displacement of the intestines (Fig. 9.5).
a. Retroperitoneal haemorrhage.
– Coagulopathy.
– Bleeding from retroperitoneal neoplasm (especially adrenal).
– Ruptured vascular anomaly.
b. Retroperitoneal urine.
– Trauma to the ureters (rupture, or avulsion from the kidneys or bladder); intravenous urography will demonstrate urine leakage, although the site of leakage may not be identified.
c. Inflammation or abscessation.
– Penetrating wounds (e.g. bites).
– Post-operative complication of ovariohysterectomy.
– Fat necrosis secondary to pancreatitis.
3. Focal retroperitoneal enlargement of soft tissue opacity.
 |
| Figure 9.18 Left renal mass on the lateral view (see Fig. 11.1 for ventrodorsal view). (From Thrall, D.E. (ed.) (1998) Textbook of Veterinary Diagnostic Radiology, 4th edition. Philadelphia: Saunders, with permission.) |
b. Enlargement of the medial iliac (sublumbar) lymph nodes ventral to L6–7; ventral displacement ± compression of the descending colon.
– Lymphoma (often with hepatosplenomegaly).
– Metastasis from malignant neoplasia in the hindquarters: prostate, urinary bladder, rectum and perianal region, pelvic canal, pelvic bones, pelvic limbs, tail.
– Reactive, secondary to inflammatory process in the hindquarters.
c. Urinoma (paraureteral or uriniferous pseudocyst) following ureteric trauma and encapsulated urine leakage.
d. Adrenal mass; mass medial or craniomedial to ipsilateral kidney; may show wispy mineralization.
– Adenocarcinoma.
– Adenoma.
– Phaeochromocytoma.
e. Abscess or focal inflammation of retroperitoneal soft tissue or sublumbar muscle.
f. Primary or metastatic neoplasia of retroperitoneal soft tissue or sublumbar muscle.
g. Soft tissue swelling associated with a lumbar or sacral vertebral lesion – look for bone changes too.
– Spondylitis.
– Spinal trauma.
– Neoplasia.
9.10. INCREASED RADIOPACITY OF THE RETROPERITONEAL SPACE AND/OR LOSS OF VISUALIZATION OF THE RETROPERITONEAL STRUCTURES
1. Soft tissue opacity of the retroperitoneal space, with loss of visualization of the kidneys and sublumbar musculature.
2. Focal mineralized opacity of the retroperitoneal space.
a. Artefactual due to blood vessels seen end on.
b. Overlying intestinal contents.
c. Incidental mineralization of adrenals in aged animals, more often in cats (bilateral, dumb-bell-shaped).
d. Ureteral calculus; intravenous urography needed to demonstrate its ureteral location, although the osmotic diuresis induced may flush the calculus into the bladder.
e. Mineralization of a tumour (e.g. adrenal tumour); especially in dogs (unilateral, wispy or patchy mineralization).
g. Mineralization of major arteries.
– Chronic renal failure.
– Vitamin D toxicosis.
– Primary hyperparathyroidism.
– Hypercalcaemia of malignancy.
– Cats – hypertension and secondary arteriosclerosis.
9.11. DECREASED RADIOPACITY OF THE RETROPERITONEAL SPACE
The dorsal part of the diaphragm and the retroperitoneal structures will be outlined.
1. Fat opacity in the retroperitoneal space.
a. Excessive sublumbar fat in an obese animal.
b. Retroperitoneal lipoma.
2. Gas lucency in the retroperitoneal space (pneumoretroperitoneum).
a. Extension of pneumomediastinum through the aortic hiatus of the diaphragm.
b. Following perineal urethrostomy or perforation of urethra during catheterization.
c. Penetrating wound.
9.12. ULTRASONOGRAPHY OF THE RETROPERITONEAL SPACE
The retroperitoneal space may be imaged from a ventral abdominal or flank approach. A high-frequency (≥ 7.5MHz) transducer should be used.
1. Retroperitoneal fluid – fluid is generally anechoic but may contain a variable number of echoes depending on the presence of cells, debris and/or gas bubbles. Fluid accumulations may be throughout the retroperitoneal space or localized. Small volumes of fluid will tend to spread along the natural planes in the retroperitoneum, giving a lacy appearance. For differential diagnoses for the causes of retroperitoneal fluid accumulation, see 9.9.2 and 9.9.3. A migrating foreign body may be seen as a hyperechoic structure, with or without acoustic shadowing, within an accumulation of fluid (see 12.6.3. and Fig. 12.5).
2. Retroperitoneal mass.
a. Neoplasm.
– Renal.
– Adrenal.
– Other.
b. Granuloma.
c. Abscess.
d. Haematoma.
– Traumatic.
– Coagulopathy.
– Bleeding from a retroperitoneal tumour or vascular anomaly.
e. Enlarged lymph nodes (see 9.13).
9.13. ULTRASONOGRAPHY OF THE LYMPH NODES IN THE RETROPERITONEAL SPACE
The medial iliac lymph nodes lie close to the abdominal aorta and CdVC at their caudal bifurcation. They may be visible in normal animals as well-defined, elongated, hypoechoic structures. The lumbar lymph nodes extend along the paralumbar tissues but are usually recognized ultrasonographically only when enlarged. It is difficult to differentiate malignant from reactive lymph nodes. Neoplastic lymph nodes are usually rounded and hypoechoic but can be heterogeneous. Doppler blood flow patterns can be helpful, with predominantly hilar flow strongly suggestive of a benign process.
1. Enlargement of lymph nodes – tend to become more rounded as they enlarge, but they may also become irregular in shape and heterogeneous in echogenicity.
a. Reactive enlargement in response to an inflammatory lesion in the hindquarters.
b. Multicentric lymphoma or other myeloproliferative disorders.
c. Metastasis from malignant neoplasia in the hindquarters.
9.14. ULTRASONOGRAPHY OF THE ABDOMINAL AORTA AND CAUDAL VENA CAVA
The aorta lies dorsal and to the left of the CdVC in the retroperitoneal space. Pulsations of both vessels may be evident, due to referred aortic pulsation affecting the CdVC. The CdVC is more easily compressed by pressure from the transducer. Doppler ultrasound allows definitive differentiation between the two.
1. Vascular intraluminal mass.
a. Thrombus or embolus secondary to:
b. Neoplastic invasion from an adjacent mass, especially adrenal.
2. Vascular narrowing.
a. Caudal vena cava, due to transducer pressure.
b. Extrinsic compression by a mass.
3. Vascular anomalies.
a. Portosystemic shunts (see 9.24).
b. Arteriovenous fistula.
c. Other anomalies (e.g. azygos continuation of the CdVC).
LIVER
Radiographic examination of the liver is often unrewarding, because the gall bladder, bile ducts and hepatic vessels are not normally detectable on plain (survey) radiographs and parenchymal changes can be suspected only when obvious focal or generalized hepatomegaly or reduction in liver size is present. Ultrasonography is of value for assessing architecture, and scintigraphy can be used for functional testing including detection of portosystemic shunts.
Assessment of liver size is best made on an RLR radiograph by noting the position of the stomach axis and the thickness of the liver between the diaphragm and abdominal structures caudal to the liver. In most breeds of dog and in cats, the gastric axis lies parallel to the 10th rib, although in deep-chested dogs it lies vertically or may even slope cranioventrally. The caudoventral edge of the left lateral liver lobe is well visualized on lateral radiographs, forming a sharp and acute angle (the hepatic angle) near the costal arch. In deep-chested dogs, the hepatic angle lies cranial to the costal arch, and in other breeds and in cats it protrudes a variable distance beyond the costal arch (up to about twice the length of L2). In dogs with a pendulous abdomen or if there is caudal displacement of the liver due to thoracic expansion, the hepatic angle will be located more caudally.
9.15. DISPLACEMENT OF THE LIVER
1. Cranial displacement of the liver.
a. Loss of integrity of the diaphragm.
– Diaphragmatic rupture or hernia.
– Peritoneopericardial hernia.
b. Enlargement of other abdominal organs, including advanced pregnancy.
c. Severe ascites.
2. Caudal displacement of the liver – expansion of thorax.
a. Iatrogenic overinflation of lungs for thoracic radiography.
b. Dyspnoea.
c. Pulmonary emphysema.
d. Large pleural effusion.
e. Large intrathoracic mass.
3. Displacement of a single liver lobe – may be obscured by abdominal effusion.
a. Lobar rupture.
b. Lobar torsion – usually larger dogs.
9.16. VARIATIONS IN LIVER SIZE
Generalized changes in liver size may be inferred from the position of the stomach (see 10.2). Liver enlargement may be due to primary liver disease or secondary to disease in another organ system; it usually has to be severe and/or extensive before changes can be detected radiographically. On lateral radiographs, generalized hepatomegaly leads to projection of the ventral part of the liver further beyond the costal arch, caudodorsal displacement of the gastric axis and an increase in the hepatic angle with rounding of the liver margins. The position of the diaphragm, stomach and spleen allows evaluation of the size of the left side of the liver. Assessment of the right side is more difficult on the lateral radiograph, although gross enlargement will displace the pylorus, cranial duodenum and right kidney caudally. Right-sided hepatomegaly is better seen on VD radiographs, displacing the stomach to the left.
Reduction in liver size may be hard to differentiate from normal deep-chested canine conformation. Signs of reduced liver size include cranial displacement of the gastric axis and transverse colon, reduced distance between the stomach and diaphragm and loss of the hepatic angle.
Normal radiographic liver size does not preclude significant liver disease.
1. Generalized liver enlargement – hepatomegaly (usually causes left-sided and caudal gastric displacement) (Fig. 9.6).
b. Young animal – liver is larger relative to body weight than in adult.
c. Venous congestion.
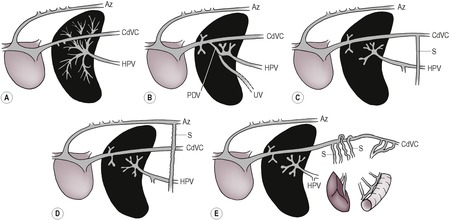
– Budd–Chiari-like syndrome (post caval syndrome); mechanical obstruction of the post-sinusoidal hepatic veins, post-hepatic CdVC or right atrium causing hepatomegaly and high-protein ascites. CdVC occlusion has various causes, including the following: compression by a diaphragmatic rupture or hernia, adhesions or kinking of the CdVC cranial to the liver (e.g. following trauma), CdVC thrombosis or invasion by neoplasia, heartworms, compression by thoracic masses, cardiac neoplasia, congenital cardiac anomalies, pericardial diseases and migrating foreign bodies.
d. Hyperadrenocorticism (Cushing’s disease) – naturally occurring or iatrogenic; may also see any or all of bronchial mineralization, pot-bellied abdomen due to muscle weakness, osteopenia, calcinosis cutis and adrenal mass.
e. Diabetes mellitus.
f. Neoplasia.
– Lymphoma (often with enlarged spleen ± lymph nodes, especially sublumbar; may also see interstitial lung pattern and thoracic lymphadenopathy).
– Mast cell infiltration (often with enlarged spleen and lymph nodes).
– Other primary and metastatic tumours.
– Malignant histiocytosis – especially Bernese Mountain Dogs, Golden and Flat-coated Retrievers and Rottweilers, ± pulmonary and hepatic masses and lymphadenopathy.
g. Severe nodular hyperplasia.
h. Hepatitis.
i. Cirrhosis – in the early stages, hepatomegaly may be seen.
j. Cholestasis.
k. Storage diseases.
l. Amyloidosis.
m. Fungal infection*.
n. Alveolar echinococcosis*.
o. Cats – hepatic lipidosis.
p. Cats – feline infectious peritonitis.
q. Cats – lymphocytic cholangitis.
r. Cats – acromegaly.
s. Cats – hypertrophic feline muscular dystrophy.
 |
| Figure 9.6 (From Thrall, D.E. (ed.) (1998) Textbook of Veterinary Diagnostic Radiology, 4th edition. Philadelphia: Saunders, with permission.) |
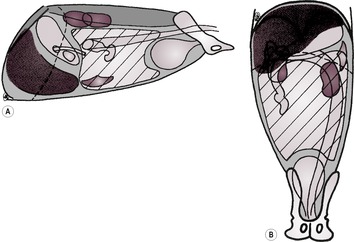
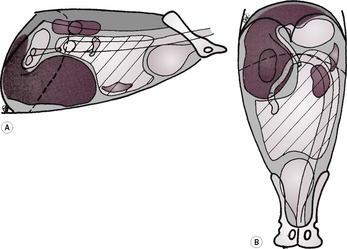
2. Focal liver enlargement (Figs 9.14 and 9.15).
a. Focal neoplasia.
– Hepatoma – may be pedunculated and lie caudal to stomach.
– Various carcinomas (e.g. hepatocellular, cholangiocellular, adenocarcinoma).
– Haemangiosarcoma.
– Lymphoma.
– Malignant histiocytosis – especially Bernese Mountain Dogs, Golden and Flat-coated Retrievers and Rottweilers; changes in other organs too, as with lymphoma.
– Metastatic neoplasia.
– Biliary cystadenoma; especially in cats.
b. Intrahepatic abscess.
c. Biliary or parenchymal cyst(s) – may be associated with polycystic kidney disease in cats.
d. Large area of hyperplastic or regenerative nodule formation.
e. Haematoma.
f. Granuloma.
g. Alveolar echinococcosis* (dogs).
h. Liver lobe torsion.
i. Biloma (biliary pseudocyst) – usually secondary to trauma or iatrogenic injury to the hepatic parenchyma.
j. Hepatic arteriovenous fistula.
 |
| Figure 9.14 (From Thrall, D.E. (ed.) (1998) Textbook of Veterinary Diagnostic Radiology, 4th edition. Philadelphia: Saunders, with permission.) |
 |
| Figure 9.15 (From Thrall, D.E. (ed.) (1998) Textbook of Veterinary Diagnostic Radiology, 4th edition. Philadelphia: Saunders, with permission.) |
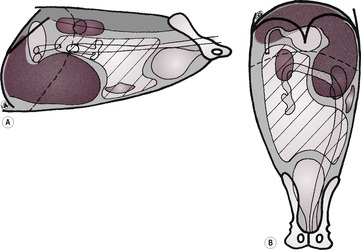
3. Reduced liver size – microhepatica (Fig. 9.7).
a. Normal radiographic appearance in deep-chested dogs.
b. Artefactual.
– Elevation of liver on large falciform fat pad in obese cats.
– Diaphragmatic rupture or hernia with liver entering the thorax.
c. Portosystemic shunt (see 9.19.1 and Figs 9.8 and 9.9) – usually diagnosed in young animals due to anomalous development of vessels associated with the hepatic portal vein; less often acquired due to portal hypertension as a result of chronic liver disease. Renomegaly, urinary tract calculi and osteomyelitis may also be present. NB: cats with portosystemic shunts often have a normal-sized liver.
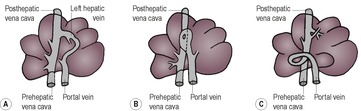 |
| Figure 9.9 (From White, R.N., Burton, C.A. and McEvoy, F.J. (1998) Surgical treatment of intrahepatic portosystemic shunts in 45 dogs. Veterinary Record142, 358–365, with permission.) |
d. Hepatic microvascular dysplasia.
e. Chronic hepatitis – various causes.
f. Chronic cirrhosis – concurrent ascites is common.
g. Idiopathic hepatic fibrosis – young dogs, especially German Shepherd dogs – ascites common.
h. Hypovolaemia (e.g. Addison’s disease).
9.17. VARIATIONS IN LIVER SHAPE