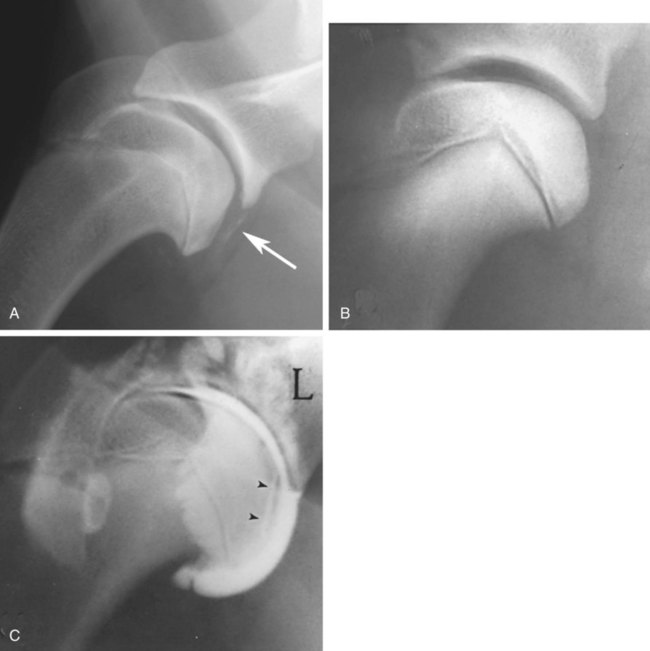
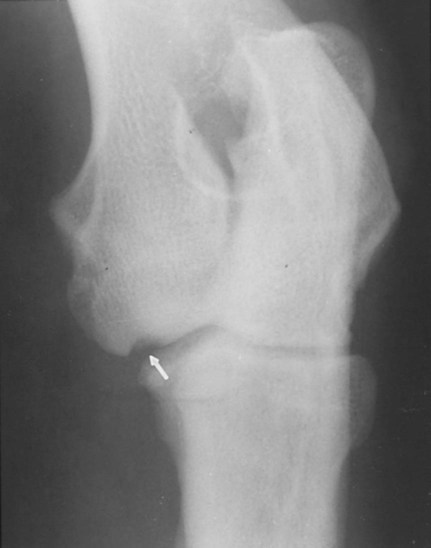
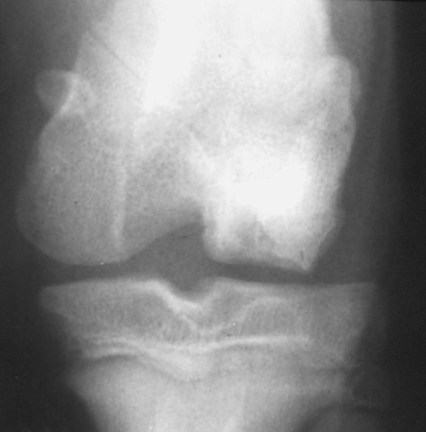
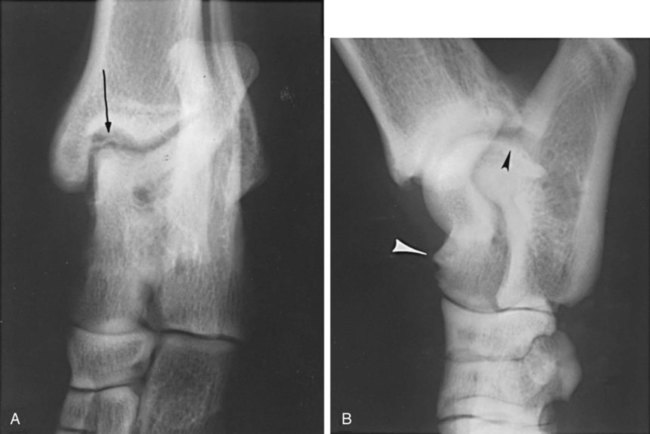
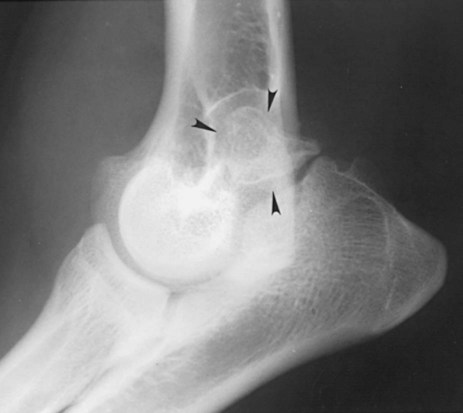
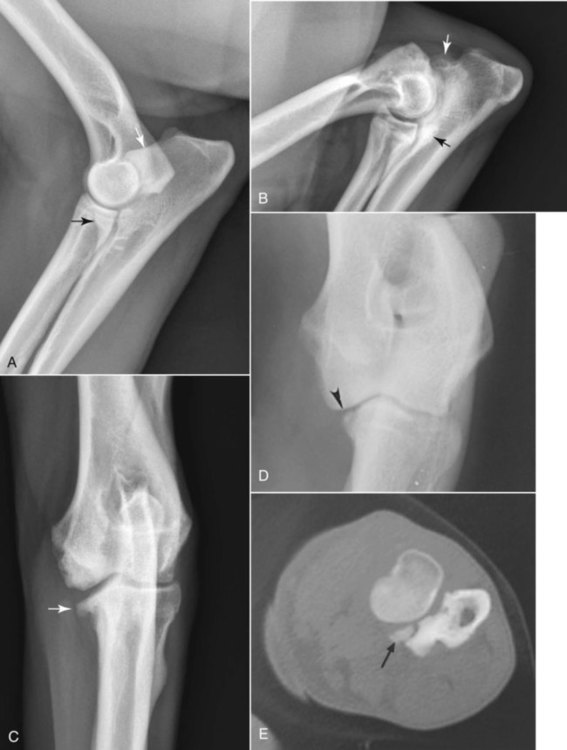
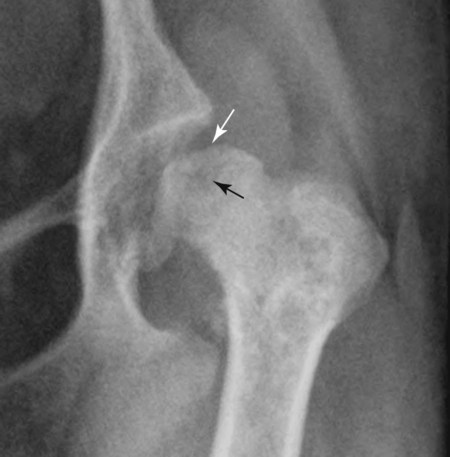
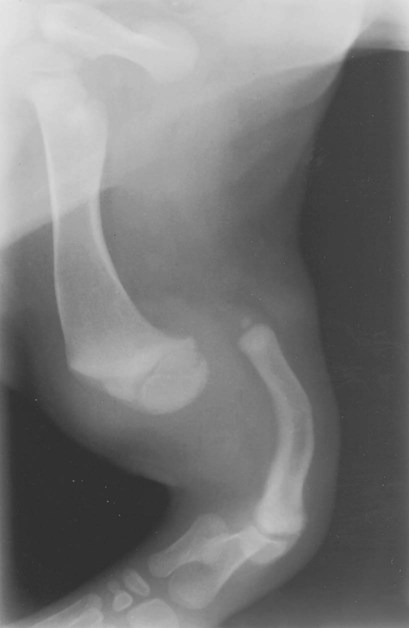
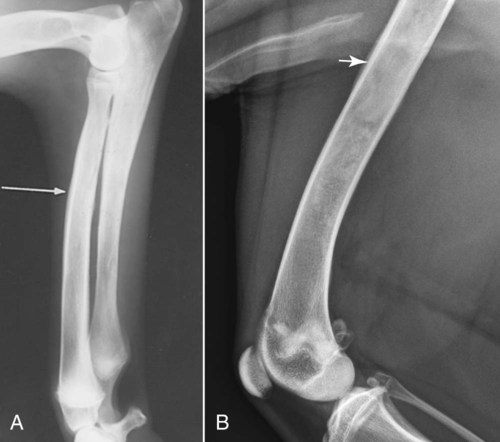
The radiographic aspects of developmental skeletal disease are as varied as the causes of the disorders themselves. Box 15-1 lists some common and uncommon disorders of the immature skeleton. It is intended to provide some structure to this chapter and should not be considered a definitive classification scheme. Osteochondrosis is a common cause of lameness in young, rapidly growing, large-breed dogs. Clinical signs usually develop between 6 and 9 months of age. Osteochondrosis occurs from epiphyseal cartilage necrosis, resulting in a failure of normal endochondral ossification.1 If the vascular bed of the adjacent subchondral bone can envelop and bypass the region of cartilage necrosis, endochondral ossification may resume without development of a clinical lesion. Otherwise, progressive chondromalacia leads to development of clefts or fissures extending from the surface of the cartilage to the subchondral bone. When a chondral or osteochondral fragment separates from adjacent subchondral bone, the disorder should technically be referred to as osteochondritis dissecans.1 However, in most patients, determination of whether a cartilage fragment exists is impossible from survey radiographs; thus osteochondrosis is an acceptable term. In dogs, osteochondrosis occurs in specific anatomic locations and often involves weight-bearing articular surfaces. It occurs most frequently in the caudal aspect of the proximal humeral head (Fig. 15-1) but also occurs in the distomedial aspect of the humeral trochlea (Fig. 15-2, p. 270), the lateral and medial femoral condyles (Fig. 15-3, see page 270), the femoral trochlea, and the medial and lateral trochlear ridges of the talus (Fig. 15-4, see page 270).2–9 Osteochondrosis is frequently bilateral, but affected animals may have clinical signs in one limb only. Large subchondral defects are frequently associated with the presence of separate osteochondral fragments, which tend to increase the severity of clinical signs.10 Gas is occasionally present within the joint space of dogs with shoulder osteochondrosis. This finding is referred to as the vacuum phenomenon and is caused by the intraarticular accumulation of nitrogen gas from negative pressure induced by traction on the joint during positioning (see Fig. 18-10). Cartilage flaps are not visible on survey radiographs unless calcification or ossification of the fragment has occurred. When a nonmineralized cartilage fragment is present, an arthrogram can be used to outline the flap if contrast medium dissects between the fragment and the underlying subchondral bone. Arthrography may also define migrating intraarticular cartilage fragments (see Fig. 15-1, C).11,12 Newer nonionic and low-osmolar contrast media provide significantly better arthrographic quality than hyperosmolar, ionic contrast media because the contrast medium is not diluted as quickly from fluid flux into the joint space.13 However, because arthroscopy has gained acceptance for the diagnosis and definitive treatment of osteochondrosis, arthrography is now used less commonly.14,15 Elbow dysplasia is a nonspecific term referring to a triad of developmental lesions that include ununited anconeal process, fragmented medial coronoid process of the ulna, and osteochondrosis of the distomedial aspect of the humeral trochlea. Although osteochondrosis has previously been implicated as the cause of all three disorders, asynchronous growth of the radius and ulna and proximal ulnar dysplasia resulting in an elliptically shaped ulnar notch have more recently been suggested as implicating factors.3,16–20 Elbow joint incongruity may result in nonuniform contact of articulating surfaces, leading to nonunion of the anconeal process or separation or fragmentation of the medial coronoid process.19,21 Although severe incongruity can be seen radiographically, computed tomography of the elbow seems to be more sensitive.22,23 One, two, or all three of the primary lesions may be present in the same animal, and both elbow joints are commonly affected. A number of breeds including German shepherd dogs, greyhounds, pit bull terriers, golden retrievers, and Labrador retrievers, are known to have a separate center of ossification at the anconeal process.24 This is typically small and poorly defined with incomplete and irregular separation from the olecranon process. Dogs with a separate center of ossification do not always develop an ununited anconeal process, and the two should not be confused with each other. The anconeal process should be fused normally to the olecranon of the ulna by 150 days of age. Breeds at greatest risk for being diagnosed with ununited anconeal process include Bernese mountain dogs, mastiffs, Rottweilers, and Saint Bernards,25 although other large-breed dogs are also at risk. A flexed lateral radiograph in addition to routine lateral and craniocaudal views of the elbow should be included in the radiographic examination. The flexed lateral view displaces the medial epicondylar physis away from the anconeus, thereby decreasing the possibility that an overlying epicondylar physeal line may be confused with an ununited anconeal process margin. The primary radiographic finding is best seen on the lateral view and consists of a radiolucent line separating the anconeal process from the olecranon in dogs older than 150 days (Fig. 15-5, see page 271). This lucent line can be sharply defined, or it may appear irregular and of variable width. Degenerative joint disease of the elbow is a common sequel, and periarticular new bone production from osteoarthrosis may partially obscure the lucency between the ulna and the anconeal process.26–28 Fragmented medial coronoid process is the most common developmental disorder involving the elbow joint. It affects medium- and large-breed dogs primarily and has a significantly higher incidence in males. Breeds at greatest risk for fragmented medial coronoid process include Bernese mountain dogs, bullmastiffs, German shepherd dogs, Irish wolfhounds, mastiffs, Rottweilers, and Saint Bernards,25 although other large-breed dogs are predisposed. Clinical signs may be apparent as early as 4 to 6 months of age. Radiographic visualization of the coronoid fragment is usually not possible because of superimposition of the medial coronoid process on the radius, superimposition of proliferative new bone from degenerative joint disease on the coronoid fragment, or failure of the x-ray beam to strike the fragment plane in a parallel fashion (Fig. 15-6, see page 272). In addition, coronoid fragments that consist mostly of cartilage or that are still partially attached cannot be seen radiographically. In most instances, the radiographic diagnosis of fragmented medial coronoid process is made indirectly through recognition of degenerative changes that accompany the primary lesion. A neutral lateral and a craniocaudal radiograph of both elbow joints should be made. In addition, a flexed lateral radiograph facilitates visualization of new bone formation on the proximal margin of the anconeal process. Flexing the elbow, however, induces a mild degree of rotation that can partially obscure the medial coronoid margin. A cranial 25-degree lateral-caudomedial view can also be obtained to highlight the medial coronoid region and fragmented coronoid process.29 Although joint incongruity can be seen in association with fragmented coronoid process, caution should be used to prevent overinterpretation of this finding. On the lateral view, the normal overlapping lucent lines representing the complex elbow joint margins can be confused with joint incongruity when even mild positioning obliquity is present. Computed tomography is now used routinely to diagnose fragmented medial coronoid process and is more sensitive than survey radiography for detecting the coronoid fragment.30–32 Primary radiographic signs include an abnormal contour, or poor definition, of the cranial margin of the medial coronoid process on the lateral view. Often the margin, which is radiographically distinct in normal dogs, cannot be followed proximally to the articular surface in affected animals. On the craniocaudal view, the medial margin of the medial coronoid process may appear blunted or rounded. A separate osseous body, representing the fractured coronoid process, is seen rarely. Joint incongruity or subluxation may also be present and appears as a stair-step lesion between the ulna and the radial head on the lateral view. Secondary radiographic signs include osteophyte formation on the proximal margin of the anconeal process as one of the earliest signs of degenerative joint disease. Similar new bone is often present on the caudal surface of the lateral epicondyle. Subchondral bone sclerosis also develops adjacent to the trochlear notch and the proximal radioulnar articulation near the lateral coronoid process. These secondary findings are best appreciated on the lateral view. A large osteophyte may arise from the medial coronoid margin on the craniocaudal view in addition to the more generalized degenerative periarticular osteophyte production.21,28,31,33,34 Aseptic necrosis of the femoral head occurs in adolescent toy and small-breed dogs (Fig. 15-7, see page 273). Compromised blood supply to the femoral capital epiphysis causes necrosis of subchondral bone while overlying articular cartilage continues to grow. Revascularization occurs in an attempt to repair the defect, and removal of necrotic bone causes decreased opacity in the affected femoral head. Incomplete removal of necrotic bone and invasion of granulation tissue interferes with healing, resulting in a misshapen femoral head of nonuniform opacity.35,36 Complete agenesis, partial agenesis, or hypoplasia of a long bone may occur. The radius, tibia, and ulna are most often involved, although the metacarpal, metatarsal, and phalangeal bones can also be affected (Fig. 15-8, see page 273). These anomalies, which can usually be detected at or shortly after birth, may be inherited but are more often a result of in utero environmental factors. Panosteitis is a self-limiting disease that affects the long bones of primarily young, large-breed dogs (Fig. 15-9, see page 273). Males are affected four times more often than are females. Breeds at greatest risk for panosteitis include basset hounds, Chinese Shar-Peis, giant schnauzers, German shepherd dogs, Great Pyrenees, and mastiffs,25 although other large-breed dogs are also at risk. Dogs between the ages of 5 and 12 months are affected most often; however, German shepherd dogs as young as 2 months and as old as 7 years have been reported. Panosteitis lesions may be solitary, affect multiple sites in a single bone, or be multifocal in multiple bones. Although the lesions can affect any part of the diaphysis of a long bone, they often originate and are most pronounced near the nutrient foramen. Bone involvement is often sequential, and the disease may be protracted over several months, with lesions resolving in some bones while developing in others. Severity and location of radiographic lesions do not necessarily correlate with the severity of clinical signs, and the most clinically affected limb may not have the most pronounced radiographic lesions.37,38 The term panosteitis is a misnomer in that histologically no evidence of an inflammatory response is present. Microscopically, medullary, endosteal, and periosteal osteoblastic and fibroblastic activity is increased.
Orthopedic Diseases of Young and Growing Dogs and Cats
Disorders Primarily Affecting Joints
Osteochondrosis and Osteochondritis Dissecans
Radiographic Signs
Elbow Dysplasia
Ununited Anconeal Process
Radiographic Signs
Fragmented Medial Coronoid Process
Radiographic Signs
Aseptic Necrosis of the Femoral Head (Legg-Calvé-Perthes Disease)
Disorders Primarily Affecting Bone
Agenesis or Malformation of Single or Multiple Bones
Agenesis and Hypoplasia
Disorders of Unknown Etiology
Panosteitis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine