CHAPTER 4 ORNAMENTAL FISH
Ornamental fish present an unusual paradox in that they are both well known and unknown to veterinarians. These animals are well known because they can be seen every day in the home aquarium, ornamental pond, pet store, and public aquarium,1 while at the same time they are unknown because knowledge regarding their health care is limited (e.g., in the areas of antibiotic residuals, antibiotic resistance, emerging diseases, antiquated or undocumented diagnostic and surgical techniques, and pain management issues). The purpose of this chapter is to address some of the current and former issues related to the health and well-being of ornamental fish.
FRESHWATER
Freshwater Temperate Fish
Most of the major taxonomic groups are represented by the freshwater temperate species.2 For many, this group represents the species commonly considered as sport fish in the United States. The most common genera of freshwater temperate fishes maintained in captivity include the sturgeon (Acipenser spp.) (Figure 4-1), paddlefish (Polyodon spathula), eels (Anguilla spp.), pike (Esox spp.), bass (Micropterus spp.), sunfish (Lepomis spp.), walleye (Sander vitreus), mullet (Mugil spp.), spotted sea trout (Cynoscion spp.), salmonids, and cyprinids. Although many of these fish are raised by hobbyists, the majority of these species require much larger systems as might be represented in a public aquarium display. Consult an introductory ichthyology text for a more detailed description of the major taxonomic groups of freshwater temperate fish.
Freshwater Tropical Fish
Freshwater tropical fish represent the largest numbers of animals typically found in home aquaria. Generally, this group of fishes is readily available for a small to moderate investment and can be easily maintained by the novice aquarist or hobbyist. Families of freshwater tropical fish include characins (e.g., tetras: Gymnocorymbus spp., Hemigrammus spp., Hyphessobrycon spp., Paracheirodon spp., Moenkhausia sp.), cyprinids (barbs: Barbus spp.), catfish (e.g., Corydoras spp., Pimelodus spp.), killifish (e.g., Aphyosemion spp., Fundulopanchax spp.), rainbowfishes (e.g., Iriatherina spp., Melanotaenia spp., Glossolepis sp.), gouramies (e.g., Colisa spp., Osphronemus spp., Trichogaster spp.), livebearers (e.g., Alfaro spp., Poecilia spp., Xiphophorus sp.), and cichlids (e.g., Pseudotropheus spp., Labidochromis spp., Iodotropheus sp., Dimidichromis sp., Copadichromis sp., Neolamprologus spp., Apistogramma spp., Microgeophagus sp., Aequidens spp., Cichlasoma spp.).2,3
Catfish
Catfish (e.g., Ictalurus spp., Lacantunia spp., Corydoras spp., Ancistrus spp., Pimelodus spp., Arius spp., Kryptopterus spp., Phractocephalus spp.) represent one of the largest groups of freshwater fishes, with more than 2000 species. Catfish have a cosmopolitan distribution. Catfish are an important group because they serve many different roles, including as ornamentals (Figure 4-2, A), as food fish in aquaculture (Figure 4-2, B), as research animals, and for sport fishing. Most catfish are found in freshwater, although there are two families that contain saltwater species.2,3 Although catfish have a cosmopolitan distribution, more than 50% of all catfish species are native to South America. There is a high degree of variability in the size and weight of these fish, with animals ranging from 10 cm to over 2 m in length and 10 g to over 300 kg in weight. Most species of catfish are nocturnal. Catfish are primarily benthic or bottom-dwellers. Because of their benthic lifestyle, catfish have sensory structures, barbels that assist them with characterizing food and nonfood items and substrate types in a low-light setting.
Cichlids
The cichlids represent one of the most diverse groups of fish, with representation in North America, Central America, South America, and Africa. These fish are prized for their diversity in size, shape, and color. African lake cichlids are one of the most evolutionarily diverse groups in the world, as many different species have evolved within limited ecologic niches (Figure 4-3). With this rapid evolution have come highly variable morphology, feeding strategies, and dietary needs. Although cichlids can look highly variable from genus to genus, they all have a common “break” in their lateral line system. The lateral line is a mechanosensory structure that assists fish with interpreting events in their environment (e.g., shifts in water pressure suggesting a predator is approaching).
Cyprinids
Goldfish (Carassius auratus), koi (Cyprinus carpio), and carp (Cyprinus spp.) are members of the largest family (>2200 species) of freshwater fish, Cyprinidae. Cyprinids have the widest area of distribution of any of the freshwater fish. Southeast Asia is the center of origin for this family of fish; however, many species have been introduced around the world and have readily adapted to these new environments.2 The carp are the largest species from this family and may hybridize with goldfish. Koi (Figure 4-4) are colorful domestic mutations of carp that are highly valued by hobbyists and the commercial pet trade. The koi can be divided into two major classifications: the nonmetallic koi and the metallic koi.2 This division is based on typical color patterns, scale types, shape, and size. Goldfish are not koi but rather descendants of the Crucian carp (Carassius carassius).2 Goldfish remain one of the most popular ornamental species and are often found in garden ponds and home aquaria. These fish generally do best if kept in cool waters (55°–68° F, 12°–20° C) with abundant plant life and adequate aeration. There are more than 30 varieties of ornamental goldfish available in the pet trade today.
SALTWATER
Many marine fish species exist, and, again, it would be impossible to cover the diversity of these fishes in a single chapter. Therefore, this chapter will focus on the species most commonly seen in captivity, including marine tropical fish, marine coldwater fish, and the elasmobranchs (e.g., sharks, skates, and rays).2–4
Marine Tropical Fish
There are approximately 23 categories of marine tropical fish. These 23 groups can be arbitrarily divided into four major groups by their feeding attributes and compatibility with other species (Figure 4-5). Most of these groups are well represented in the aquarium trade and in public aquaria.
The first category of marine tropical fish is the “rapid eater” and includes the angelfish (e.g., Pomacanthus spp.), damselfish (e.g., Amblyglyphidodon spp.), squirrelfish (e.g., Holocentrus spp.), triggerfish (e.g., Balistapus spp., Balistoides spp.), and groupers (e.g., Cephalopholis spp., Variola spp.).2 These fish do well if kept at low densities in the aquarium or if they are provided a large area where overcrowding is not an issue (e.g., large reef tanks in public displays). Hostile interaction is a major concern for these animals, as they can be very food aggressive.2–4 These animals may be kept together quite readily if provided an abundance of food and a diverse diet. Often they are kept in live coral reef tanks with anemones and other species of animals from the same group.2,3
The slow eaters represent the largest subgroup of marine tropical fish. This group includes, but is not limited to, the anemone clown fish (e.g., Amphiprion ocellaris), parrotfish (e.g., Sparisoma spp.), puffers (e.g., Carinotetraodon spp., Tetraodon spp., Colomesus spp.), surgeonfish (e.g., Acanthurus sohal), wrasses (e.g., Cheilinus spp., Halichoeres spp., Hemigymnus spp., Thalassoma spp.), and trunkfish (e.g., Aulostomus spp., Strophiurichthys spp., Ostracion spp.). Compatibility is an issue with this group of animals, and they do best if maintained in large displays with a large amount of hiding area. Although these fish are grouped based on their feeding strategy, there remains a great deal of variability in the diets of these animals.
The last group of animals to be categorized as marine tropicals includes the snappers (e.g., Lutjanus spp., Pristipomoides spp., Nemadactylus spp., Etelis spp.) and grunts (e.g., Haemulon spp.). These are reasonably large, territorial schooling fish that are, for the most part, kept at public facilities. They can best be described as “gluttons,” not only for the manner in which they feed but also for the almost insatiable hunger they exhibit. They are virtually fearless in their feeding habits and will attempt to remove food from the jaws of even large predators.2
Elasmobranchs
There are more than 350 species of sharks in the world, and only a small percentage are represented as display animals or kept by private aquarists and hobbyists. The species that are found occasionally in home aquaria include the carpet sharks (e.g., Parascyllium spp.) (Figure 4-6), catsharks (e.g., Galeus spp., Scyliorhinus spp.), and horned sharks (e.g., Heterodontus spp.). Most of the other species of sharks are much too large to be kept in anything less than a public aquarium or large commercial facility. Many public facilities display saw sharks (e.g., Pristiophorus spp.) and multiple species of ornamental sharks, including angel sharks (e.g., Squatina australis), dogfish (e.g., Squalus spp., Scymnodon spp., Deania spp., Centroscymnus spp.), and frilled sharks (e.g., Chlamydoselachus spp.).
Skates and rays are closely related to sharks; however, they are markedly different in appearance (Figure 4-7). There are both freshwater and saltwater varieties of stingrays. The skates and rays are dorsoventrally flattened animals and usually have at least one venomous spine on the dorsal caudal fin (tail). There are multiple freshwater species (Figure 4-8) that are small enough to be kept by the private aquarist; however, the majority of these animals are also found in public facilities. There are a number of ornamental species of saltwater rays, such as the guitarfish (e.g., Rhinobatos spp.), butterfly rays (e.g., Gymnura spp.), and cow-nose rays (e.g., Rhinoptera spp.), that have been maintained and successfully reproduced in public facilities. Many of the larger, saltwater rays, such as the southern stingray (Dasyatis americana), Atlantic stingray (Dasyatis sabina), eagle ray (Myliobatis aquila), and giant manta ray (Manta birostris), are also now being kept in public aquaria.
UNIQUE ANATOMY AND PHYSIOLOGY
When veterinarians begin to work with a new species of animal, it is imperative that they develop a basic understanding of the animal’s anatomy and physiology. A background knowledge of anatomy and physiology will prove beneficial when collecting diagnostic samples or administering therapeutics. The following is a review of unique anatomic and physiologic features of fish. (See other resources for additional information regarding this subject.2)
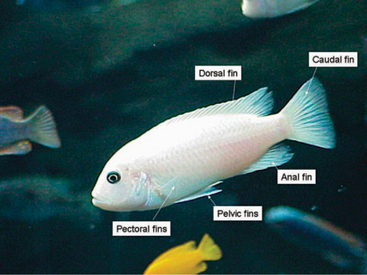
Teleosts typically have two sets of paired fins (e.g., pectoral and pelvic) and three unpaired fins (e.g., dorsal, anal, and caudal) (Figure 4-9). Fins are used for steering, balancing, and braking. Certain species have modified fins to adapt to certain niches. For example, the anal fin of the knifefish is a large, single fin located on the ventrum of the animal. This fins serves as the animal’s primary source of locomotion. Spines may be associated with some fins and serve as a defense mechanism. The lionfish (Pterois volitans) produces venom that can be injected into a potential predator, causing significant pain and discomfort. Knowledge of the species that produce venom is essential to prevent injury to the handler. Fish may damage their spines when captured in a net. To prevent this, fish may be scooped into a plastic cup or bucket to facilitate removal from the aquarium.
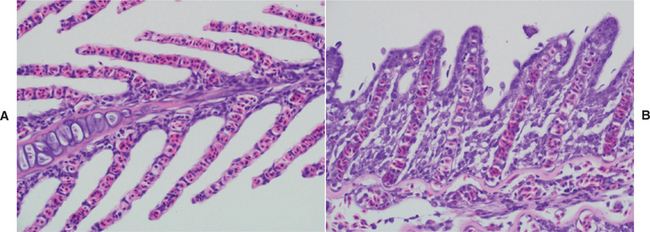
The respiratory system of fish is vastly different from the respiratory systems of higher vertebrates (e.g., reptiles, birds, and mammals). Gills are the primary respiratory organs of most fish, although certain species use accessory organs to aid in the absorption of oxygen. Gills serve to absorb oxygen, excrete waste products (e.g., ammonia and carbon dioxide), and regulate ion and water balance. Teleosts have four pairs of gills; elasmobranchs can have five to seven pairs. The gills are attached to a bony gill arch, and each gill is comprised of primary and secondary lamellae. The secondary lamellae are the site of gas exchange (Figure 4-10). Exposure to parasites and toxic compounds, such as ammonia, results in excessive production of mucus, which can impede gas exchange. The presenting symptoms of affected animals include rapid opercular movements and gulping for air at the water’s surface; among these animals sudden death may occur.
The fish kidney is a single structure that is comprised of two segments: anterior and posterior. The kidney is located dorsal to the swim bladder along the body wall. The fish kidney serves as both an osmoregulatory and a hematopoietic organ. The anterior kidney and the interstitium of the posterior segment serve as the primary sites for blood cell and immunoglobulin production in fish, as these animals do not have bone marrow. The posterior kidney primarily regulates electrolyte and urine output. Fish that are found in saltwater (hypertonic) environments tend to lose water and absorb salts. To prevent dehydration, these fish must drink water and excrete excess electrolytes, such as sodium and chloride, through the kidney and gills. Fish that live in freshwater (hypotonic) environments constantly absorb water by osmosis. To prevent overhydration, freshwater fish excrete large volumes of dilute urine.
HUSBANDRY
Environmental Considerations
It is easy to recognize aquatic facilities (institutional or pet retail) that have the most disease problems on the basis of their appearance. There is usually an accumulation of trash, dirty exhibits, dead animals, and a general “unhealthy” appearance to the collection. This does not mean that a “clean” facility is free of disease but that they traditionally have fewer and less severe outbreaks of disease.5 The general husbandry and maintenance of the aquatic facility and ecosystems have a direct relationship to the overall health of the animals. Maintaining clean areas behind the exhibits and nonpublic areas is essential to good health practices.3,5 The same can be said for retail aquarium facilities. Accumulation of feces, excess food, and detritus are predisposing factors to poor water quality and can serve as substrates for facultative pathogens. Cleaning and disinfecting seines, nets, buckets, and tanks are imperative to maintain a high-quality health program at a facility. A dynamic team effort is required to maintain a clean facility and healthy animals, but a clean facility will pay dividends by providing a safer workplace, reduced disease incidence, increased production, and generally healthier fish.
Given ideal environmental conditions, ecosystems require a certain amount of space to fully develop, though quantitatively how much space is a debatable matter. Generally, to place an ecosystem in a very large aquarium or other holding space is not a major ecologic problem, although it might be considered an engineering endeavor. The difficulty arises when veterinarians attempt to scale down and include many components in a much smaller space than which would normally occur in the wild. To miniaturize an ecosystem and place it into a small space for observation, education, or research, veterinarians are immediately faced with a major dilemma, which is to scale the miniature so that it can still function as a reasonable facsimile of the wild ecosystem. This question is intimately related to the entire problem of how veterinarians affect wild environments and how they restore them, as well as how they construct their aquaria.5,6
Creating an Aquarium
There is little biologic reason for the traditional “box type” aquarium shape; however, the common reason for its existence is associated with availability and mechanical, aesthetic, and economic convenience (Figure 4-11). For many scientists and aquarists, the ease of purchase and setup of a ready-made tank outweighs all other factors when a water-based system is desired.
The presence of tank walls that can support benthic communities or allow excessive light is undesirable. A weakly translucent cylindrical tank that minimizes attachment surface for a given volume and has a rotating, cleaning mechanism to keep the surface free of sediment is highly desirable but very expensive.
TANK SIZE
Choosing the correct size of tank is an easy task. The main principle to remember is that bigger really is better. The smallest tank size we recommend is the commercially available 20-gallon tank (24″ × 12″ × 20″; 61 cm × 30.5 cm × 50.8 cm); however, the larger tanks provide an even more stable environment for fish. A larger tank also will offer the benefit of a much more liberal air-to-water surface area for the fish. However, with the advancements in life support technology today, it is possible to provide more than adequate life support to maintain large densities of fish in smaller aquaria while also maintaining a greater diversity of animals.4,7,8
TEMPERATURE
The term tropical places too much emphasis on the idea of “high temperature” for all exotic fishes. A number of these ornamental fish are not from the tropics, and quite a few from the tropics do not come from particularly warm water. It is important to recognize that a large number of exotic ornamental fish cannot thrive in cool, chilly water (<68° F or 20° C), because it affects their metabolic rate or immune function; other species cannot thrive in extremely warm water (>86° F or 30° C), because they need more oxygen than the water has the ability to carry. There is no exact degree of heat that is best suited to each species. Most fish tolerate a 10° F fluctuation over time and can stand a 5° F change in a short period of time (e.g., minutes) without consequence.4,5 It is almost impossible to find a place in nature where the temperature falls into the controlled ranges that veterinarians try to achieve in the captive environment, and it would be reasonable to believe that temperature changes can be beneficial and stimulating to the animals. In practice, the aquarium environment should be geared toward the temperature needs of the species of fish that will inhabit the aquarium. It is best to create fish communities from similar ecosystems and attempt to maintain the temperature range as close as possible to the native waters of those particular species. Temperature changes will occur, but if they occur within the basic guidelines mentioned previously, these changes will be beneficial to the well-being of the animals.
FILTRATION
Mechanical Filtration
Mechanical filtration represents one of the original forms of filtration used in the home aquarium. This type of filtration removes organic debris from the water by passing it through a filter material (e.g., floss, fiber, or paper cartridge) (Figure 4-12). The amount of filtration that can be accomplished using this type of filter depends on the type and size of the filter material and rate that the water is recirculated through the filter media. A densely packed fiber or small pore size will restrict the size of waste that can pass the filter media, resulting in less waste in the aquarium. Maintenance of these filters requires cleaning or replacement of the floss or cartridge. Mechanical filtration remains an important method of filtration in home aquaria, outdoor ponds, and public aquaria. This type of filtration is best used with other types of filtration (biologic or chemical) to improve the overall quality of water in the system. When a series of filters is used inline, it is best to place the mechanical filter first. This ensures that the heavy organic material will be removed before contaminating or clogging the other filters (e.g., chemical or biologic-sand filter). Mechanical filtration does have limitations; for example, it is not effective in trapping finite particles or chemicals.
Biologic Filtration
Ammonia is the primary end product of protein catabolism in fish. In a closed system, this waste product can be fatal to fish. It was the advent of biologic filtration that led to our ability to maintain large densities of fish in small volumes of water. Biologic filtration is the most common type of filtration used in the home aquarium and outdoor pond, and it comes in many different forms (e.g., under-gravel filters, bio-wheels, sand or bead filters, and wet-dry filters) (Figure 4-13). A biologic filter should be selected based upon the expected load on the system. If a large density of fish is going to be maintained in a system or the fish are going to be fed large quantities of food to ensure growth (e.g., aquaculture), then a large surface area for bacteria is needed.
LIGHTING
Correct lighting for the aquarium system depends on several factors, including the quality of the light emitted from the selected light source. Full-spectrum lighting is preferred. This type of lighting provides the three primary light spectrums: ultraviolet, visible, and infrared. The various spectrums can be affected by water depth, with certain spectrums (e.g., ultraviolet) being removed in the epilimnion (upper water level). Another important factor is the function of the system. If the tank is going to house deep-water African cichlids, lighting becomes less important; however, if the lighting system is needed for a reef tank that is going to be stocked with corals, a significant quantity of high-quality full-spectrum light will be required. The primary disadvantages associated with excess lighting are algae overgrowth and overheating the water in smaller systems; otherwise, it is virtually impossible to have too much light in an aquarium system.4 A 12 hour photoperiod is considered appropriate for most fish. For those individuals interested in breeding fish, the photoperiod should be lengthened to mimic a spring/summer season.
SUBSTRATE
Historically, aquarists have tended to ignore the substrate of an aquatic system, reducing it to a noninteracting element. In the aquaria of past decades, “clean,” relatively inert gravel and under-gravel filters were used to provide environments for all but the most specialized natural situations. In nature, with the exception of gravel bottoms in relatively unproductive hard rock mountain streams or sandy beaches with sandstone composition, rarely is the substrate material neutral. In most aquatic and marine environments, soft substrates are rich in organic reservoirs and harbor a myriad of important invertebrates and microbes that support rich plant growth. Limestone substrates control water chemistry, and reef corals and rocks determine the very character of the organisms growing on the surface of the reef. The interest in so-called live rock in coral reef tanks in recent years is beginning a tendency to replace sterile environments with live ecosystems. The addition of “trickle trays” with calcium carbonate pebbles also shows a developing interest in further elucidating the carbonate cycle and control over the pH. Conversely, acid, black water streams are most likely to occur in granite or sandstone areas where the natural acidity of the rain and tannic acid from the forest litter cannot be neutralized. To recreate this environment, the aquarist is advised to use hard rock and silica sand. Coarse sand or gravel is perhaps the most difficult of benthic environments for organisms to adapt to, and within sand and gravel habitats there are relatively few common species. In a stream or small lake that is not large enough for significant wave activity, or in a bay or coastal lagoon along a sandy coast, the sediment becomes progressively finer from gravel, to sand and silt, to a soupy, silty-clay mud substrate. To remain sand, the bottom must stay in constant motion; therefore, special adaptations are required by any organism to adapt to it. Even bacterial numbers tend to be limited in sand and gravel because their organic substrates are often washed out.9,10 It can be difficult to maintain a sandy, shore style substrate in a closed, captive system, as it can have a rather long profile in the energy regime required to maintain the sand. It would be impossible to recreate a wave-break, sandy scenario within a miniature system unless the benthic community is the only one desired.
ACCESSORIES FOR AQUARIA
There are a myriad of commercial accessories available for aquaria today. These accessories range from simple items, such as heaters and aeration devices, to some of the most advanced biotechnology available in the field, such as chillers and wave machines. Most commercial accessories available on the market are well made, and the deficiencies have been minimized over the years. When choosing a particular item, selection should be based on function not brand. For example, with a heater, it is important to match the wattage of the instrument to the volume of water to be heated, taking into account the general temperature required for the environment in which the aquarium is to be located.4 There are several types of heaters available, including basic submersible heating elements, solar units, flow through units, or a combination of similar systems.
WATER QUALITY ANALYSIS
The aquatic environment is a very complex system that is subject to constant physical and chemical changes. A water-assessment program should be devised for monitoring an aquatic ecosystem. Many factors need to be taken into consideration when designing a water quality monitoring program, including the volume of water in the aquarium/aquaria, the number and type of animals that will be present, the type of life support system, and the period of time the aquarium has been set up. Ideally, a water-assessment program should be established before designing and setting up a system, keeping in mind that most of the water quality analysis is done to keep its inhabitants healthy.8
Water testing should be done daily for new aquaria until the system has cycled. When collecting a water sample, it is important to collect a mid-water sample, as samples closer to the surface or substrate will reflect more extreme values.2,3 Portable water analysis kits use premeasured reagents and simple analytic methods that may compromise the degree of accuracy. However, they are suitable for most private aquarists and small backyard ponds. Most large, public aquaria use more advanced analytic methods, such as mass spectrometry, saturometers, and sophisticated chemical analysis, for evaluating water quality. The acquisition and utilization of many of these processes and techniques are beyond the scope of what most private aquarists can afford. Commercial water quality laboratories can perform a complete water analysis for a modest price, and it may be a good idea to submit a sample to a laboratory for a baseline measurement. This method also offers the added benefit of comparing standardized values with the parameters derived from veterinarians’ own testing methods.
Ammonia, Nitrite, and Nitrate
Ammonia is produced in fish as an end product of protein catabolism. This waste product is primarily excreted via the gills, although some excretion in the feces also occurs. Ammonia is also generated in the aquatic system from the breakdown of uneaten food and detritus. Ammonia nitrogen can occur in two forms: ammonium (NH4+) and ammonia (NH3). Ammonia is the more toxic form for fish.11 The relative concentration of each form varies with pH and water temperature.12 Ammonia is soluble in water, and minimal amounts are lost through evaporation. In a closed system, such as an aquarium or backyard pond, ammonia levels can build up to toxic quantities (>1 ppm). Even low levels of ammonia can be toxic to the gills and skin, resulting in increased susceptibility to infections. Fish suffering from ammonia toxicity appear irritated, gasp at the water’s surface, and rub against rocks in the aquarium as a result of the irritation caused by the toxin. Ammonia levels should be monitored closely in closed systems, especially those with high fish densities. Testing for ammonia should be done weekly using a standardized commercial test kit, which is available at local pet retailers. In an established system, ammonia levels should be zero. If the ammonia levels begin to rise, then the system should be reevaluated. Overfeeding and overstocking an aquarium can overburden the biologic filter. Severe temperature fluctuations and insufficient oxygen levels may also result in a significant loss of the biologic filter. This is especially common in ponds that have significant summer algal blooms. New systems require time to become established, and new fish should be added gradually to prevent an overload of the biologic filter.
Ammonia and nitrite levels in an aquatic system may rise soon after treatment of the water with antibacterial compounds or a reduction in water temperature. Antibiotics added to the water are nonselective and may kill both pathogenic and commensal organisms. If these compounds kill enough of the bacteria associated with the biologic filter, then oxidation of ammonia and nitrite may stop. Nitrosomonas spp. are more temperature tolerant and will recolonize before Nitrobacter spp., so ammonia levels should be expected to decrease before nitrites. Therefore, elevated nitrite levels are often detected soon after a reduction in water temperature.
Hardness
Hardness measures the quantity of divalent cations (e.g., calcium and magnesium) in the water. In natural waters, the divalent ions are derived from the limestone, salts, and soils. The general range for hardness in freshwater systems is 0 to 250 mg/L, whereas in saltwater systems, hardness can exceed 10,000 mg/L.13 The divalent cations that represent hardness, calcium and magnesium, play an intricate role in water quality conditions. Calcium can alter osmoregulatory function in fish during times of low pH or elevated ammonia.13 Calcium and magnesium are both important for growth and development in fish fry.14 These minerals can also protect fish against heavy metals by competing for gill absorption sites. Copper is routinely used to treat parasites. Calcium and magnesium compete with the copper for absorption sites, reducing the effectiveness of the copper. Distilled water should never be used to replenish water in an aquarium because it is deficient in these essential cations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree