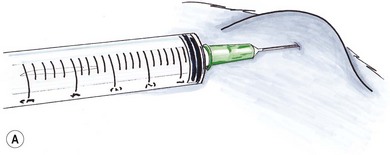
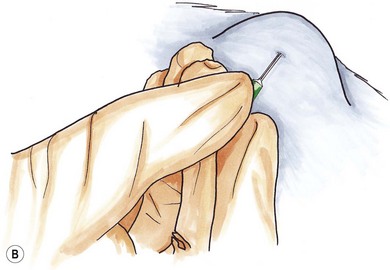
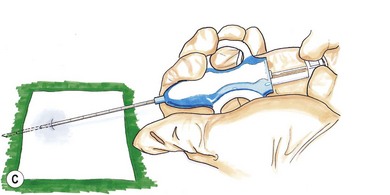
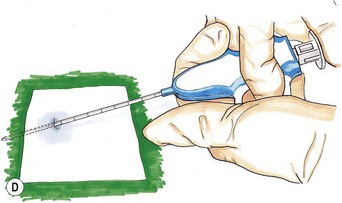
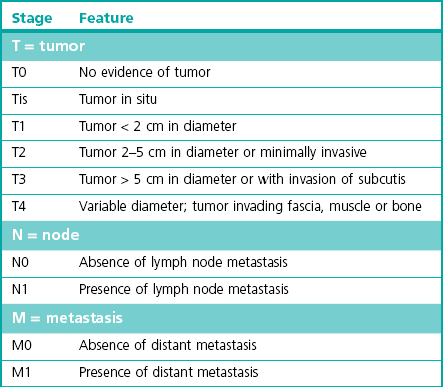
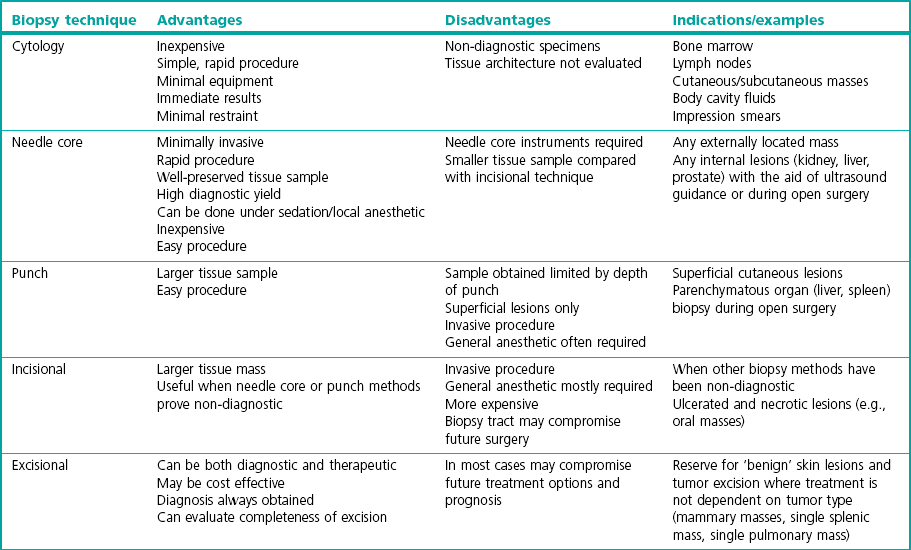
Chapter 14 Cancer is known to be an important disease entity in the cat, affecting all age groups. Although a current incidence rate of neoplasia in this species is not available, past data from a US tumor registry report suggests an overall occurrence of 0.5%. Although malignant tumor incidence rates were similar to dogs, the incidence of benign tumors in cats was ten times less than that of dogs.1 A more recent animal registry from two areas in Italy provided an estimated annual incidence rate of 0.77% for cats. Furthermore, cats had a 4.6-fold higher incidence of malignant than benign tumors, and purebred cats had an almost 2-fold higher incidence of malignant tumors than mixed breeds. Tumor incidence increased with age in the cat populations.2 With the emergence of oncology as a veterinary specialization and increased public interest in pet cancer it is unsurprising that treatment of feline cancer patients is now commonplace, in both the general practice setting and specialist centres. Diagnostic imaging techniques form a crucial part in the assessment of the cancer patient. It is important to recognize the rapid growth of more sophisticated modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and nuclear imaging (e.g., scintigraphy) and the reader is referred to Chapter 8 for more information. Conventional radiography is still commonly used, partly due to financial reasons, particularly for the assessment of pulmonary metastasis. Ultrasonography is a useful complementary tool and can provide important information regarding tumor location, character and local invasion. CT provides excellent evaluation of bony lesions and is the gold standard for the evaluation of pulmonary metastasis. Three-dimensional reconstruction greatly facilitates surgical planning, and integration of CT scans with radiotherapy allows much more precise and targeted delivery of radiation to the patient (Chapter 15). MRI offers excellent soft tissue contrast and is ideal for evaluation of the brain. Availability and finances are often the two main reasons that limit the routine use of these imaging modalities in general practice. Rhinoscopy for visualizing nasal tumors, cystoscopy for assessing bladder neoplasia and thoracoscopy for examining pulmonary or mediastinal masses are examples of minimally invasive techniques that can combine both presurgical tumor assessment and biopsy procurement in one procedure. Details of these techniques are discussed in Chapters 8 and 24. Before treatment can be considered it is important to define the precise extent of the tumor burden of each patient, by means of clinical and histological staging. This provides valuable information not only for treatment planning, but also helps evaluate response to treatment and disease progression. Staging is based on the World Health Organization Program on Comparative Oncology TNM (tumor, node, metastasis) system, with several adaptations for a variety of tumors of domestic animals. An example of such an adaptation is illustrated in Table 14-1 for feline tumors of epidermal origin (carcinomas). Not all tumors lend themselves to staging easily, and in some tumors, with feline mammary tumors being a prime example, it has been shown that the tumor histological grade is significantly linked to prognosis and can be used as an independent prognostic factor.3,4 Table 14-1 World Health Organization classification for feline tumors of epidermal origin (Modified with permission from Owen LN. Classification of Tumors in Domestic Animals. WHO. http://whqlibdoc.who.int/hq/1980/VPH_CMO_80.20_eng.pdf) Obtaining a diagnosis is one of the most important steps in the management of the cancer patient. Obtaining a biopsy before the surgical procedure is performed is best clinical practice in the majority of cases as it provides a pre-treatment diagnosis, helps the clinician plan the surgery, and can provide the owner with a more accurate prognosis. There are a number of methods for obtaining samples from the tumor and the choice is based on a number of factors including tumor location, suspected tumor type, safety of the procedure, the patient’s clinical status, cost, equipment availability, and surgeon’s preference. With the exception of diagnostic cytology, all other techniques listed in Table 14-2 involve tissue sampling and histological interpretation. Table 14-2 Comparison of different biopsy techniques (Reprinted from Demetriou JL, Foale RD. Small Animal Oncology. London: Saunders Elsevier; 2010, with permission from Elsevier.) Fine needle aspiration (FNA) is a cost-effective, simple first-line option to obtain a diagnosis from a wide variety of masses, either on the skin or within body cavities. Enlarged, accessible lymph nodes are easily aspirated and a major advantage of using this method over the histological methods of biopsy is that the sample can be examined ‘in-house’ after appropriate staining. The equipment that is required is listed in Box 14-1. Aspiration can be performed using either the needle attached to the syringe or the ‘capillary’ method, which involves inserting just the needle without the exertion of negative pressure. The act of briskly redirecting the needle hub backwards and forwards within the mass is enough for cells to become detached and move up the core of the needle. This is the author’s preferred technique as it is less likely to result in excessive fluid being aspirated that would effectively dilute the cellular component to the sample. Box 14-2 illustrates the steps involved in FNA using both the methods described. There have been several studies evaluating the accuracy of FNA involving cats. One study found that in ultrasound-guided samples of splenic masses, cytologic diagnoses (by FNA) corresponded with histologic diagnoses in 61% of cases.5 In another study involving only four cats in which liver aspirates were taken, false results were obtained in all cases when compared with surgical biopsy samples. In these cases hepatic lipidosis was diagnosed by aspiration of samples that were later confirmed as inflammatory or neoplastic.6 However, in another study of canine and feline patients results suggested that FNA may be a sensitive and specific method of evaluating the regional lymph nodes in dogs and cats with solid tumors, because results correlated well with results of histologic examination of the entire lymph node.7 In conclusion, these results suggest that the accuracy of FNA may vary considerably and this may be partly dependent on the tissue being sampled. Regional lymph node aspiration appears to be highly specific and sensitive. CT-guided aspiration of bone and lung tissue has also been reported in cats and studies suggest that it may be a safe and accurate procedure.8,9 Needle core biopsy provides a quick and easy way of obtaining a tissue sample. It can be used on externally or internally (usually in combination with ultrasound guidance) located masses and can be performed using local anesthetic and sedation, providing a cost effective and less invasive option to clients compared with incisional biopsy. As the initial incision and tract made by the instrument is small, there is little risk of disruption of the tumor and subsequent tumor seeding, although removal of the biopsy tract is recommended at the definitive surgery. Although designed as disposable instruments, they can be reused after sterilization with ethylene oxide, and performing the skin incision with a scalpel blade before insertion of the needle will help delay blunting of the tip and extend the life span of the instrument. In core biopsy the typical needle size is 18–14G and comprises an inner notched stylet with an outer cannula (e.g., Tru-cut). Box 14-3 illustrates the steps involved in obtaining a needle core biopsy sample. In one study there was 96% agreement with presurgical Tru-cut results and postsurgical biopsy results when 48 diagnostic tumor samples from dogs and cats were evaluated.10 The results indicated that needle core biopsy can accurately predict surgical biopsy. Thus, needle core biopsy performed before surgical excision of masses can facilitate planning and reduce the need for numerous surgical procedures.
Oncological surgery
Evaluation of the cancer patient
Imaging
Minimally invasive techniques
Staging
Biopsy techniques
Fine needle aspiration
Needle core biopsy
Oncological surgery