Fig. 1
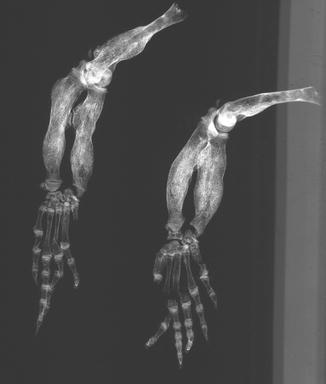
Displaced fracture in fossil frog BMS F9W-133; middle Holocene, Hiscock Site, Byron, western New York, USA (courtesy of Michael Grenier and Richard Laub)

Fig. 2
Polarizing microscopic view of uric acid crystals responsible for gout
Fusion (of unknown significance) between vertebrae (Fig. 2) has been reported in Pipa, Dactyletra, Systoma, Ceratophrys, Brachycephalus, Palaeobatrachus, Brachycephalus, Rana esculenta, Bufo cinereus, and with the urostyle, in Bufo variabilis, Pipa, Dactyletra, Systoma, Phyllomedusa, and Pelobates (Adolphi 1893, 1895, 1898). Reports of spinal pathology have since been limited to scoliosis (Bacon et al. 2006; Ouellet 2000), with the exception of the observations of Shilton et al. (2008). Their notation of intervertebral space loss in cane toad Bufo marinus invading Australia is associated with irregular hyaline cartilage islands and new bone formation, characteristic of neuropathic arthritis (Rothschild and Martin 2006).
Neoplasia is equally rare. Frequent (11.5%) Inkiapo frog Rana chensinensis enchondromas (and a single chondrosarcoma) were attributed by Green and Harshbarger (2001) to paper factory waste. Lathyrism-induced chondymyxomas in Pacific chorus frogs Pseudacris regilla and Rana fusca and chordoma in hybrid toads were attributed to ingestion of sweet pea (Lathyrus odorata) by Green and Harshbarger (2001).
Pathology in Extinct Amphibians
Reports in ancient amphibians are limited to congenital (homeobox) phenomenon and isolated fractures. Other pathologies are rarely observed but have not been sufficiently described and analyzed for etiology to be confidently recognized.
Midline skull ossifications or atypical sutures (Table 1) were reported among nasals, frontals, parietals, and postparietals in 4–12% (Gubin and Novikov, 1999). The pineal foramen was absent in Thoosuchus yakovlevi. Frontals were laterally expanded in Thoosuchus yakovlevi and included in orbit borders in Platyoposaurus watsoni, Konzhukovia vetusta, Benthosuchus korobkovi, and Melosaurus platyrhinus.
Table 1
Skull ossification and suture anomalies in fossil amphibians
Apateon | Eryops megacephalus | Osteophorus roemeri |
Aphaneramma | Gonioglyptus | Platyoposaurus |
Batrachosuchoides | Kestrosaurus | Schoenfelderpeton |
Benthosuchus | Konzhukovia | Sclerocephalus |
Lydekkerina | Thoosuchus | |
Branchierpeton | Melanerpeton | Trematosuchus |
Chelyderpeton | Micropholis | Volgasaurus |
Cochleosaurus | Mordex calliprepes | Wetlugasaurus |
Edops | Onchiodon |
Reported post-cranial anomalies among extinct amphibians have been limited to the axial skeleton. Sacral anomaly and fusion with adjacent vertebrae or urostyle (Figs. 3 and 4) were noted in Holocene frogs (Böhme 1982) and unilateral sacral wing enlargement, in Palaeobatrachus luedeckei and diluvianus (Špinar 1972). Witzmann (2007) reported a Middle Triassic temnospondyl with a hemivertebrae, restricted dorsal aspect of second pleurocentrum and larger alternate side intercentrum.


Fig. 3
Congenital fusion of vertebrae in fossil frog BMS E11SW-123; middle Holocene, Hiscock Site, Byron, western New York, USA (courtesy of Michael Grenier and Richard Laub)

Fig. 4
Pygostyle-vertebral fusion with periosteal reaction along pygostyle in fossil frog BMS F6SW-115; middle Holocene, Hiscock Site, Byron, western New York, USA (courtesy of Michael Grenier and Richard Laub)
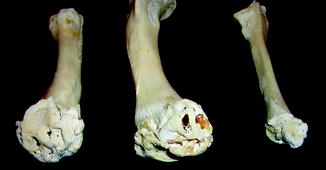
Reported femoral and tibial fractures affected Holocene Rana sp. and Bufo cf. bufo (Ippen and Heinrich 1977). The latter were infected (Fig. 5). Fractured, bowed fibulare was fused to tibiale in Palaeobatrachus (Špinar 1972). An exostosis which would have hosted a cartilaginous cap (osteochondroma) in a fossil frog is illustrated in Fig. 7.

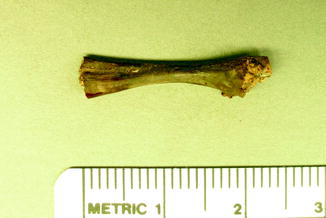

Fig. 5
Infectious vertebral fusion in fossil frog BMS G8SW-182; middle Holocene, Hiscock Site, Byron, western New York, USA (courtesy of Michael Grenier and Richard Laub)

Fig. 6
Congenital anomaly in sacrum of fossil frog BMS F8NE-241; middle Holocene, Hiscock Site, Byron, western New York, USA (courtesy of Michael Grenier and Richard Laub)

Fig. 7
Osteochondroma in fossil frog BMS F5SE-162; middle Holocene, Hiscock Site, Byron, western New York, USA (courtesy of Michael Grenier and Richard Laub)
Pathology in Contemporary Reptiles, Exclusive of Turtles
Fractures of the lower jaw, rib, and leg are described in lizards and crocodiles (Figs. 8 and 9); vertebrae and ribs, of snakes (Abel 1935; Anthony and Serra 1951; Dämmrich 1985; Isenbügel and Frank 1985; Reichenbach-Klinke 1963; Rothschild 2008; Schlumberger 1958). Focal elevation of periosteum (Figs. 10–14) identifies hematoma secondary to trauma.
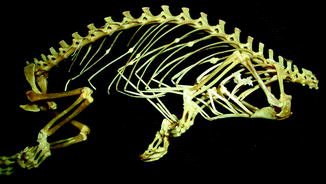
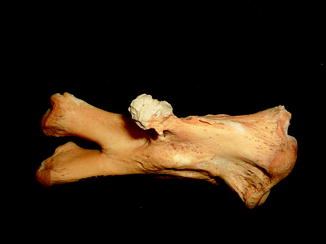
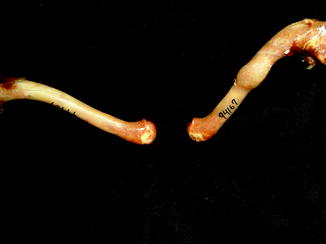
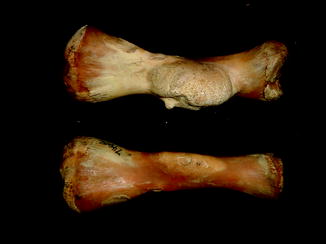
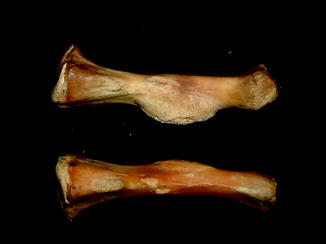
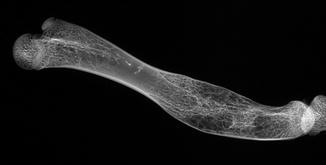

Fig. 8
Lateral view of panther chameleon Furcifer pardalis (YPM 13662) skeleton with multiple rib fractures

Fig. 9
Healed fracture with exostoses, with apparent cartilage cap, characteristic of osteochondroma; ventral view of tibia and fibula of Crocodylus niloticus (NMNH 42150)

Fig. 10
Large smooth focal bone enlargement, characteristic of hematomas; lateral view of tibia of Varanus komodoensis (AMNH 74606)

Fig. 11
Isolated bone bump identifies hematoma in Iguana iguana (AMNH 94167)

Fig. 12
Hematoma; face view of Varanus komodiensis (AMNH 79606)

Fig. 13
Hematoma; lateral view of Varanus komodiensis (AMNH 79606)

Fig. 14
Lateral x-ray view of Iguana iguana ROM 42979 humerus. Periosteal expansion around hematoma
Bicephalism is the most commonly reported congenital pathology in snakes and has occasionally been noted in lizards (Acharji 1945; Amaral 1927; Cunningham 1937; Matz 1989; Payen 1991; Wallach 2004). Their congenital origin contrasts with tail duplications, which appear to be post-traumatic events (Blair 1960). Isolated reports of conjoined twins include the slow worm (Anguis fragilis), sand lizard (Lacerta agilis), and blue-tongued skink (Tiliqua scincoides) (Barten 1996; Bellairs 1965; Frye 1991; Hildebrand 1938). Other congenital anomalies (Figs. 11, 15–17) (Table 2) are usually reported as isolated phenomenon (Kälin 1937), but some (e.g., polymely, ectomely, and ectodactyly) have been attributed to environmental factors (Johnson et al. 2003; Ouellet et al. 1997; Rostand 1951, 1960).


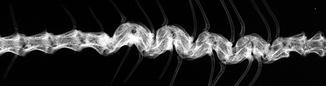

Fig. 15
Divot in articular surfaces, characteristic of osteochondrosis; face view of articular surfaces of Crocodylus sp. (NMNH 15r5?82)

Fig. 16
Congenital vertebral fusion producing a spiral pattern; dorsal view of vertebrae of Iguana iguana (ROM 42980)

Fig. 17
Dorsal-ventral x-ray view of Iguana iguana ROM 42980 vertebral column in Fig. 16. Congenital deformity
Table 2
Anomalies in reptilesa
Brachycephaly – New Guinea crocodiles, Alligator, Crocodylus niloticus, Crocodylus porosus |
Cleft palate – C. porosus Lacerta lepida, Lacerta vivipara, Varanus exanthematicus Vipera berus |
Protruding mandible – Crocodylus porosus, C. johnsonii |
Snout curvature – gharial |
False nostrils – C. johnsonii |
Tail shortening/absence – Crocodylus niloticus, Osteolaemus tetraspis |
Supernumerary limb – crocodile |
Rib/vertebral duplication – Aelodon priscus, Rhacheosaurus gracilis. Alligator, Crocodylus acutus, Heloderma horridum and suspectum |
Hemivertebrae – African rock python Python sebae |
Intercalated half-vertebrab – African rock python Python sebae, Burmese, Indian or Ceylon Python molurus, Tropidonotus, Pelamis platura, Gavialis, Coluber, Diadophis punctatus, Natrix natrix, Elaphe, Lampropeltis, Rhadinacea, and Thamnophis |
Ectromelia – Crocodylus moreletii and caiman |
Vertebral osteomyelitis is the most common infection in snakes (Ippen 1965; Isaza et al. 2000). Multifocal granulomatous spondylitis was noted in the hybrid Crotalus mitchellii/willardi (Frye and Kiel 1985). Infection, although less common, is often multifocal (Figs. 18–28) in other reptiles (e.g., Alligator mississippiensis, Caiman latirostris, and Crocodylus siamensis) (Brown 1971). Other isolated reports include an infected shoulder in Alligator mississippiensis (Frye 1991) and Erysipelothrix in Crocodylus acutus (Jacobson 2007).
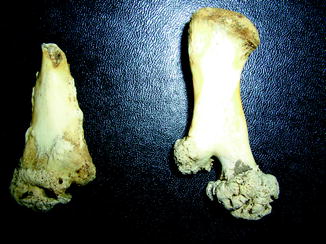
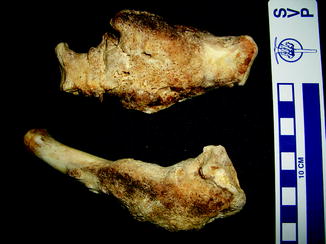
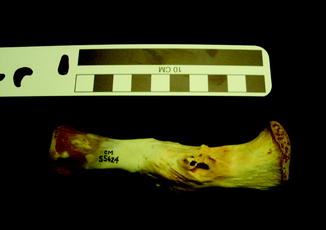
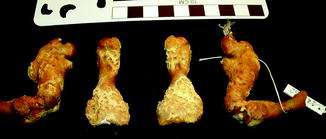
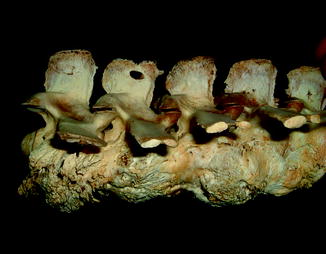

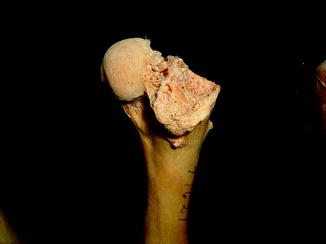




Fig. 18
Destructive changes with new bone formation, characteristic of infection; ventral view of femur and tibia of Alligator mississippiensis (UFMNH 34788)

Fig. 19
Gross bone enlargement and distortion with filigree reaction by infectious process; lateral view of limb bones of Alligator mississippiensis (KU 19538)

Fig. 20
Draining sinus of osteomyelitis; lateral view of tibia of black caiman Melanosuchus niger (CM 55624)

Fig. 21
Surface disruption characteristic of infection; anterior view of femora and humeri of Varanus exanthematicus (CM 14052)

Fig. 22
Reactive new bone with draining sinuses, characteristic of infectious spondylitis; lateral view of vertebrae of Alligator mississippiensis (KU 19538)

Fig. 23
Reactive new bone with draining sinuses, characteristic of infectious spondylitis; ventral view of vertebrae of Alligator mississippiensis (KU 19538)

Fig. 24
Severe destructive changes characteristic of infection; lateral view of proximal femur of Alligator mississipiensis (AMNH 71621)

Fig. 25
Bony overgrowth around infectious process; ventral view of sacrum and lumbar vertebrae of Varanus komodoensis (UMMZ 236581, with 11,876 = Field number)

Fig. 26
Destruction by infectious process; en face view of sacrum and lumbar vertebra of Varanus komodoensis (UMMZ 236581)

Fig. 27
Surface defect probable from draining sinus; ventral view of phytosaurid (NMNH 18313)
Osteoarthritis is essentially, a disease of captivity (Fig. 29), and extremely rare in the wild (Frye 1991). Metabolic disease (Table 3) in reptiles presents as articular gout, pseudogout (calcium pyrophosphate deposition disease), and metabolic bone disease (Figs. 30 and 31). The latter, essentially limited to captive reptiles (Frye 1991), presents as pathologic fractures and folding fractures (Barten 1996; Frye 1973). Calcific overgrowths, noted in boa constrictors, rattlesnakes, a Burmese python (Python molurus bivittatus) (Frye 1991), and in a varanid (Figs. 32–39), are of unclear diagnosis. While they consist of wormian bone, the diagnosis of osteitis deformans or Paget’s disease has been discarded (Jacobson 2007).
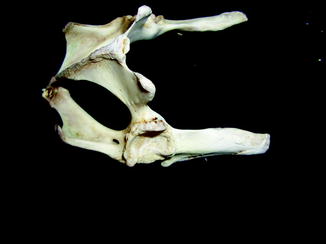
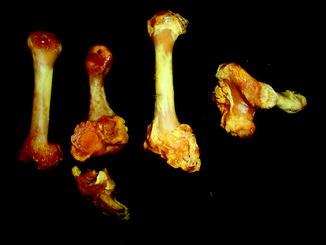
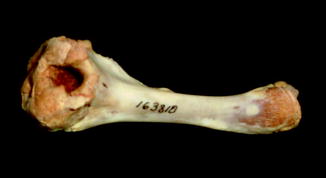
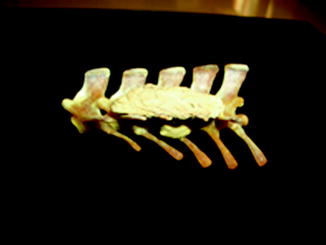

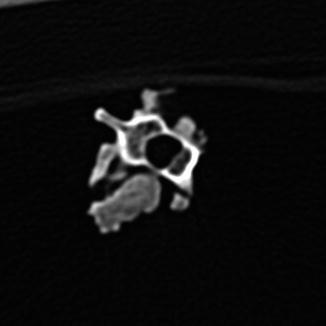
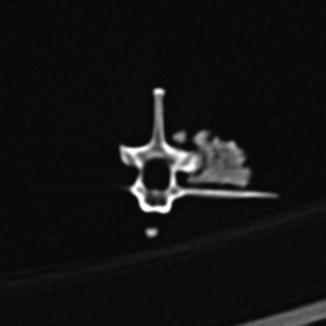
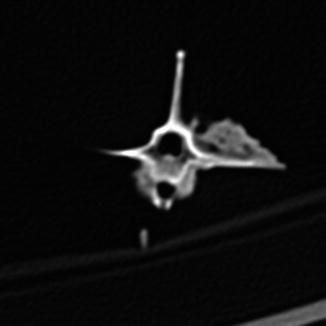
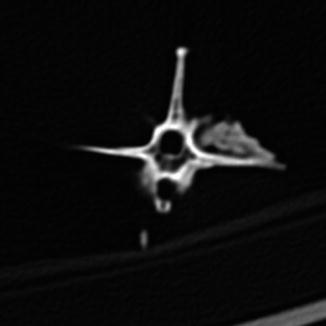
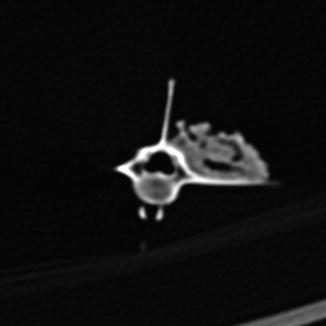
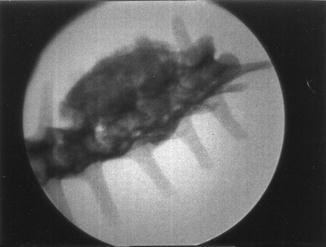

Fig. 29
Overgrowth of bone (osteophytes) around left acetabular joint, characteristic of osteoarthritis; ventral-oblique view of pelvis of Varanus salvator (UFMNH 33136)

Fig. 30
Calcium deposits on and about joint surfaces; lateral view of metacarpals and carpals of Varanus komodoensis (YPM 11626)

Fig. 31
Ventral view of Varanus salvator (NMNH 163810). Hole in bone cause by gout

Fig. 32
Mass of bony tissue adjacent to ribs; lateral view of string of vertebrae of Varanus dorianus (UMMZ 238879)

Fig. 33
Mass of bony tissue adjacent to ribs; dorsal view of vertebrae of Varanus dorianus (UMMZ 238879)

Fig. 34
Mass of bony tissue adjacent to ribs, with varying degrees of separation; computerized tomographic view of vertebrae of Varanus dorianus (UMMZ 238879)

Fig. 35
Mass of bony tissue adjacent to ribs, with varying degrees of separation; computerized tomographic view of vertebrae of Varanus dorianus (UMMZ 238879)

Fig. 36
Mass of bony tissue adjacent to ribs, with varying degrees of separation; computerized tomographic view of vertebrae of Varanus dorianus (UMMZ 238879)

Fig. 37
Mass of bony tissue adjacent to ribs, with varying degrees of separation; computerized tomographic view of vertebrae of Varanus dorianus (UMMZ 238879)

Fig. 38
Mass of bony tissue adjacent to ribs with varying degrees of separation; computerized tomographic vertebrae view of Varanus dorianus (UMMZ 238879)

Fig. 39
Dorsal-ventral x-ray view of Varanus dorianus (UMMZ 238879) vertebrae. Mass of bony tissue adjacent to ribs. Disruption of adjacent internal vertebral centra architecture, characteristic of osteomyelitis
Table 3
Crystalline arthritis
Articular Gout – Varanus exanthematicus, Tupinambis, Iguana iguana, Caiman sclerops, spectacled caiman, Alligator mississippiensis, Crocodilus acutus, C. moreletii, C. novaeguineae, C. johnsonii, Nile crocodiles, Indo-Pacific crocodile, false gharial and gharial, Tomistoma schlegelii, Gavialis gangeticus |
Pseudogout – Egyptian spiny-tailed lizard Uromastyx – Varanus komodiensis |
Examination of a number of reports of exostoses in Iguana iguana (Chiodini and Nielsen 1983) or Paget’s disease in a boa constrictor, Bitis nasicornis, Crotalus, and southern copperheads (Isenbügel and Frank 1985) has revealed a new diagnosis of the inflammatory arthritis of the spondyloarthropathy variety (Rothschild 2009) and infection. Diffuse periosteal reaction may represent infection or hypertrophic osteoarthropathy (Figs. 40 and 41). A form of inflammatory arthritis associated with vertebral fusion and peripheral and axial joint erosions with reactive new bone and joint fusion (Figs. 42–53) has been documented in 2–20% of Varanus, dependent upon species (Rothschild 2009), representing the disease category termed spondyloarthropathy. A crystal arthritis, related to deposition of calcium, has been noted in varanids (Figs. 54–61) (Rothschild 2010).
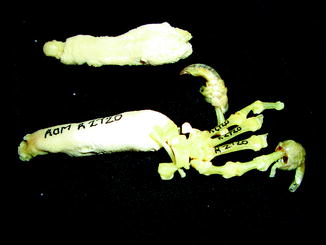
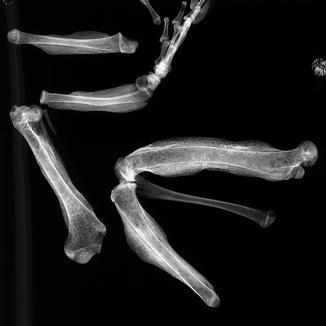

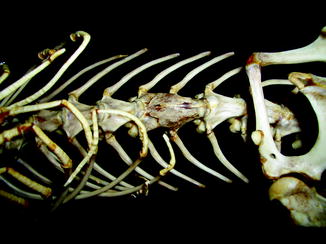

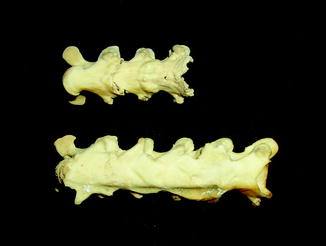
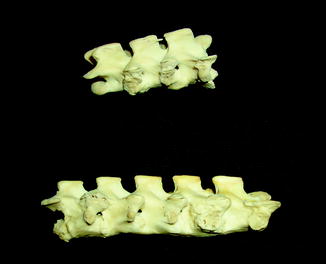
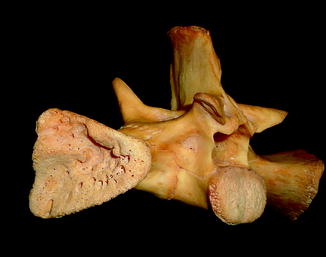
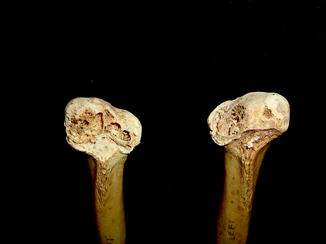
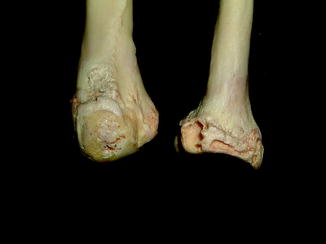
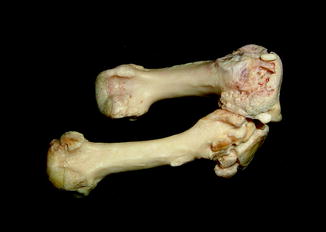
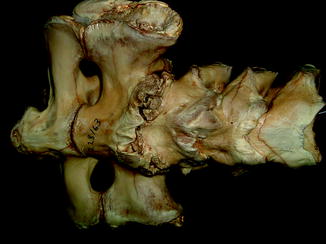

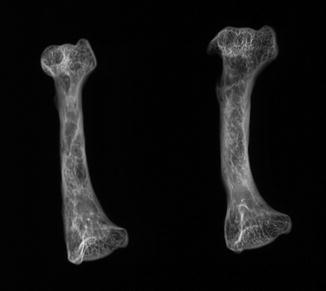
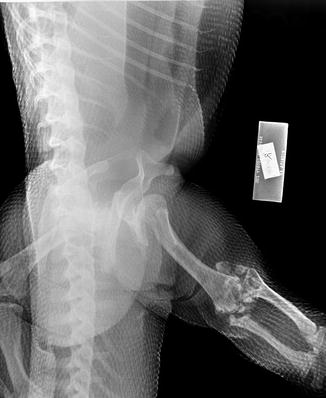
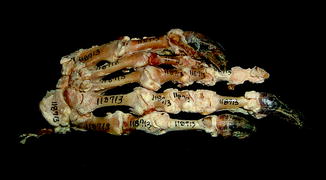
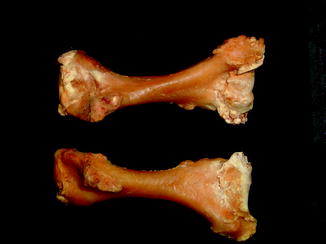
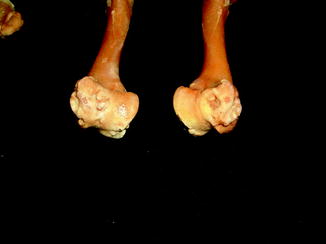
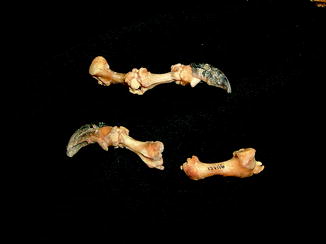
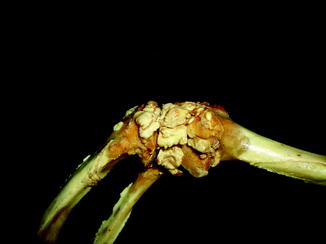
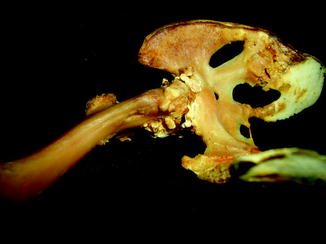
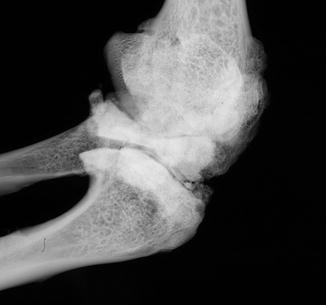

Fig. 40
Diffuse periosteal reaction; ventral (upper) and dorsal (lower) view of distal limbs of Iguana iguana (ROM R2720)

Fig. 41
Lateral x-ray view of Iguana iguana ROM 42981 extremities. Generalized periosteal reaction from hypertrophic osteoarthropathy

Fig. 42
Destruction of zygapophyseal (facet) joint by erosive process; anterior view of vertebra of Alligator mississippiensis (KU 19538)

Fig. 43
Smooth vertebral bridging by syndesmophyte, characteristic of spondyloarthropathy; ventral view of caiman lizard Dracaena guianensis (YPM 10224) skeleton

Fig. 44
Smooth vertebral bridging by syndesmophyte, characteristic of spondyloarthropathy; lateral view of caiman lizard Dracaera guianensis (YPM 10224) skeleton

Fig. 45
Smooth bridging of vertebral centra, characteristic of spondyloarthropathy; ventral view of thoracic vertebrae of Varanus albigularis (YPM R 13717)

Fig. 46
Smooth bridging of vertebral centra, characteristic of spondyloarthropathy; lateral view of thoracic vertebrae of Varanus albigularis (YPM R 13717)

Fig. 47
Erosive process characteristic of spondyloarthropathy; face view of facet joint of Varanus olivaceus (UMMZ 218977)

Fig. 48
Erosive process involving subchondral bone, characteristic of spondyloarthropathy; ventral view of two femora of Varanus olivaceus (UMMZ 218977)

Fig. 49
Subchondral and large marginal erosions with reactive new bone formation, characteristic of spondyloarthropathy; lateral view of distal femur and distal tibia of Crocodilus acutus (AMNH 7121)

Fig. 50
Subchondral erosions characteristic of spondyloarthropathy; superior-ventral view of metatarsals of Varanus komodoensis (ROM 7565)

Fig. 51
Bridging of vertebral bodies, with subtle sacral erosions, characteristic of spondyloarthropathy; ventral view of vertebrae and sacrum of Varanus komodoensis (NMNH 228163)

Fig. 52
Dorsal view of Varanus komodoensis (UMMZ 236581) vertebrae. Zygapophyseal joint fusion characteristic of spondyloarthropathy

Fig. 53
Dorsal-ventral x-ray view of stump-tailed skink Trachydosaurus rugosus ROM 23461 humerus. Erosion with sclerotic margin

Fig. 54
Articular crumbling with calcium deposits, identifying calcium pyrophosphate deposition disease; dorsoventral x-ray view of right knee of Varanus komodoensis (Sedgwick County Zoo 6668)

Fig. 55
Calcium deposition around joints, characteristic of calcium pyrophosphate deposition disease; dorsal view of manus of Varanus bengalensis (AMNH 118713)

Fig. 56
Calcium deposition around joints, characteristic of calcium pyrophosphate deposition disease; posterior view of humeri of Varanus olivaceus (UMMZ 218977)

Fig. 57
Ill-defined, crumbling articular surface erosions with calcium deposition, characteristic of calcium pyrophosphate deposition disease; posterior view of distal ends of humeri of Varanus olivaceus (UMMZ 218977)

Fig. 58
Calcium deposition around joints, characteristic of calcium pyrophosphate deposition disease; lateral view of digits of Varanus niloticus (AMNH R 137116)

Fig. 59
Calcium deposition around joints, characteristic of calcium pyrophosphate deposition disease; lateral view of the knee of Varanus salvator (ROM 23468)

Fig. 60
Calcium deposition around joints, characteristic of calcium pyrophosphate deposition disease; inferior-oblique view of pelvis and femur of Varanus salvator (ROM 23468)

Fig. 61
Lateral x-ray view of Varanus salvator ROM 23468 knee. Cartilage calcification from calcium pyrophosphate deposition disease
Neoplasia has been directly observed in the form of benign growths such as enchondroma, osteoma (Fig. 63), osteochondroma (Figs. 9, 63–65), and chondro-osteofibromas and malignant growths (Fig. 66), as well as indirectly identified because of their induction of hypertrophic osteoarthropathy (Figs. 41 and 67) (Table 4).
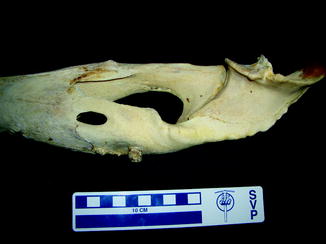
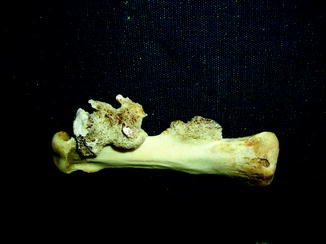
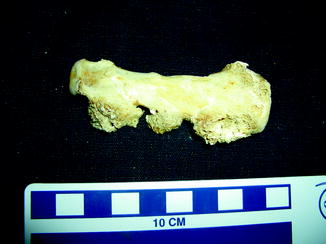
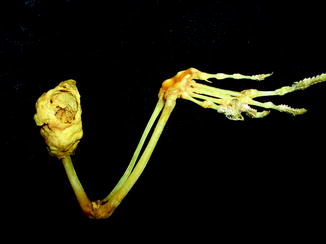


Fig. 63
Small bone overgrowth identifies osteoma; medial view of right mandible of Alligator mississippiensis (KU 19538)


Fig. 65
Exostotic bone overgrowth with cartilage cap (osteochondroma); medial view of metatarsal of Alligator mississippiensis (KU 19538)

Fig. 66
Large proximal humeral mass with large spherical proximal defect; lateral view of lower extremity of Hypsilurus dilophus (YPM R 11404)

Fig. 67
Diffuse bone enlargement by hypertrophic osteoarthropathy; lateral view of Iguana iguana (ROM 42981) lower extremity
Table 4
Neoplasia in reptilesa
Benign | |
Enchondroma | Varanus dracaena, Varanus indicus |
Osteoma | Varanus salvator, Lacerta viridis, Uromastyx hardwickii |
Osteochondroma | Varanus bengalensis, V. niloticus, Iguana iguana, Cyclura nubila, Uromastyx hardwicki, Naja nigricollis |
Chondro-osteofibroma | Cyclura cornuta, Lacerta viridis, African rock python Python sebae |
Malignant | |
Chondrosarcoma | Elaphe guttata |
Osteosarcoma | Lampropeltis zonata, Molurus bivittatus, Rhamphiophis rostratus, Lacerta viridis, Iguana iguana |
Osteochondrosarcoma | Naja nigricollis |
Metastatic | |
Melanoma | Pituophis melanoleucus |
Squamous cell | Tupinambis teguixin |
Hypertrophic | |
osteoarthropathy | Iguana iguana |
Extinct Reptile Pathology, Exclusive of Turtles and Dinosaurs
Congenital anomalies are rarely reported. Witzmann (2007) reported a congenital hemivertebra was observed in a Jurassic Cimoliasaurus plicatus (Witzmann 2007), and Hopley (2001) reported Schmorl’s nodes (vertebral endplate holes) in a plesiosaur. MacGregor (1906) noted that the odontoid is fused with axis centrum in Phytosauria. Tooth malposition is present in Pseudosuchia (Fig. 68).
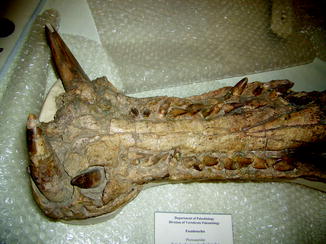

Fig. 68
Malignment of teeth; dorsal view of mandible of Pseudosuchia
Injuries have been reported predominately as isolated observations. These include fractures and exostoses (Figs. 69–71). The latter are found especially on lower jaws, vertebrae (especially caudal), and paddles (especially digits). Williston (1898) reported distal caudal vertebral fractures with pseudoarthroses. The exception is Sawyer and Erickson’s 1998 study of Leidyosuchus formidabilis, which documented gastralia as the most common fracture site.
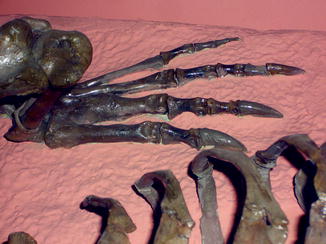

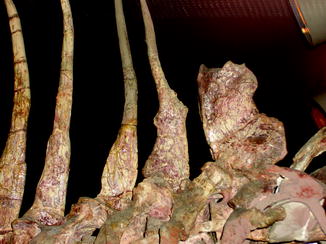

Fig. 69
Healed second metatarsal; dorsal view of hindfoot of Crocodylus clarus (NMNH 12719)

Fig. 70
Fractured neural spines; lateral view of Permian Dimetrodon grandis (NMNH 8635)

Fig. 71
Lateral view of Permian Dimetrodon grandis (NMNH 8635) spinous processes. Healed fracture with callus formation
Decompression syndrome (bends) is universally recognized on the basis of linear patterns of bone loss or articular surface subsidence (Fig. 72) in Platecarpus, Tylosaurus, Mosasaurus, Plioplatecarpus, Prognathodon, Hainosaurus and in an Antarctic mosasaur, and invariably absent from Clidastes, Ectenosaurus, Globidens, Halisaurus, and Kolposaurus (Rothschild and Martin 1987, 2006). With the exception of the large percentage of the skeleton afflicted in Platecarpus, the frequencies of vertebral involvement in the other affected genera were indistinguishable (p < 0.02). Among the basal portion of the family Sauropterygia, avascular necrosis was found in only a single genus, Neusticosaurus (Rothschild and Storrs 2003). It is seen in both early and late Plesiosauridae, Elasmosauridae. Large intergeneric variation in frequency was noted among ichthyosaurs (Montani and Rothschild, 1999). Ophthalmosaurus, Ichthyosaurus, Temnodontosaurus, and Platypterygius equally affected; Leptonectes, less so (chi-square = 5.36, p < 0.025) (Montani and Rothschild 1999), while Stenopterygius was spared.
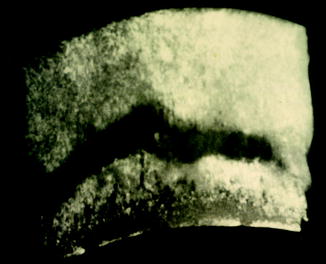

Fig. 72
Radiolucent zone transects vertebra, identifying avascular necrosis; dorsoventral x-ray view of Late Cretaceous Platecarpus (KU 152143) vertebra
Infections are noted, predominantly as isolated phenomenon (Fig. 27). Auer (1909) suggested that changes in a Metriorhynchus cf. moreli femur represented fungal infection.
The only report of osteoarthritis in a fossil reptile was in the wrist and metacarpal phalangeal joints of pterodactyloid pterosaurs (Bennett 2003). Spondyloarthropathy has been observed in Dimetrodon, Diadectes, and Ctenorhachis (Figs. 73–75).

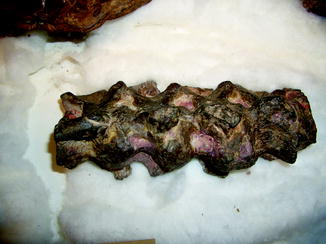

Fig. 73
Syndesmophyte bridging the centermost vertebrae, characteristic of spondyloarthropathy; lateral view of vertebrae of Early Permian Ctenorhachis jacksoni (NMNH 437711)

Fig. 74
Bridging of vertebral centra and facet joints, characteristic of spondyloarthropathy; lateral view of vertebral column of Early Permian Dimetrodon incisivus (NMNH 6723)

Fig. 75
Note anterior bony bridging indicative of spondyloarthropathy; lateral view of vertebrae of Early Permian Diadectes (NMNH)
Moodie’s 1918 diagnosis of an osteoma in a mosasaur has been reassessed as another form of benign tumor, a hamartoma. The latter is actually overgrowth of normal tissue and not truly a neoplasia. Sawey and Erickson (1998) are credited with the first report of an actual osteoma and also described a probable chondroma in Eocene Crocodylus sp.
Vertebral fusion is well recognized in the fossil record. While the pathology in some mosasaurs is clearly related to the trauma of shark bites (Moodie 1917), other mosasaurs have vertebral bridging more characteristic of spondyloarthropathy (Figs. 76 and 77), as has been noted in contemporary varanids (Rothschild 2009). Ruffer’s 1917 description and illustrations of a Lower Miocene crocodile Tomistoma dowsoni and those of Sawyer and Erickson (1998) of Leidyosuchus formidabilis clearly document spondyloarthropathy. Pales’ 1930 description of anulus fibrosus ossification in a Pleistocene Cuban saurian further documents the antiquity of the problem.



Fig. 76
Lateral view of fused Late Cretaceous mosasaur (AUMP PR 164) vertebrae

Fig. 77
Cross section of fused Late Cretaceous mosasaur (AUMP PR 164) vertebrae illustrated in Fig. 76
Pathology in turtles (Reprinted from Rothschild BM, Schultze H-P, Pellegrini R. in press. Osseous and articular pathologies in turtles and abnormalities of mineral deposition. In: Morphology and Evolution of Turtles, D Brinkman, J Gardner, P Holroyd (eds.). New York: Springer):
It is difficult at times to determine whether anomalies observed in the embryo or neonate are the result of genetic alterations or of exposure to adverse environmental conditions, or to distinguish anomalies that have been inherited versus those occurring secondary to a new mutation. One does not necessarily need to invoke an environmental toxin. Environmental conditions may be just as important, either as a direct or indirect effect. Lynn and Ullrich (1950) examined turtle embryos from eggs exposed to partial drying during gestation interval days 35–50. Embryo pathologies ranged from “almost unrecognizable carapaces and plastrons, distorted limbs, and eyeless or jawless heads.” Newman (1923) suggested temperature effect, whereas Coker (1910) considered this as an effect of egg distension. Packard et al. (1977) noted that prolonged exposure to temperatures several degrees below the optimum produced developmental abnormalities. Incubation of the California desert tortoise, Gopherus agassizii, above the optimum temperature of 32 °C was associated with upper jaw foreshortening and lack of forelimbs (Frye 1991c), whereas incubation at 33.5°C was associated with anophthalmy, maxillofacial clefts, harelips, forelimb and partial hind limb agenesis, coccygeal hypoplasia and scute and shell plate duplication (Frye 1989, 1991c). However, organochlorine compounds (e.g., PCBs) induce missing claws and eyes, deformed carapaces, tails, limbs and crania, deformed upper and lower jaws in snapping turtles (Bishop et al. 1989). Greatest incidence of deformities was noted in Cranberry Marsh. Frequency in Lake Ontario was greater than in Lake Erie or Algonquin Park, correlated with organochlorine levels.
Whereas most studies represent isolated reports of deformities (e.g., Tables 5–9), several report statistical results. Bellairs (1981) and Yntema (1960) reported that 15% of the common snapping turtle, Chelydra serpentina, had abnormal tail, hind limb, or carapace. Carswell and Lewis (2003) reported 0.32% abnormalities in 14,361 loggerhead Caretta caretta turtle eggs in 1992 and 2001, manifest as small size, carapace deformities, folded or forshortened flippers, misshapened mouthparts and twinning. The most complex of the congenital anomalies in turtles is perhaps the Siamese, conjoined or parasitic twin (Anonymous 2007a; Kuvano 1902; Maddux 1996; Nakamura 1938). Most have been reported as isolated phenomena (Table 5). A variety of duplication patterns have been observed: plastron to plastron, side to side, or posterior to posterior. Noting Hildebrand’s (1938) tally of 27 cases, Table 5 suggests the rarity of this phenomenon. Only 45 cases appear to have been reported to date, and no epidemiologic studies are available. Complete or partial absence of a limb has been reported in isolated occurrences (Table 6). Not all are congenital. Jackson (1980) noted one instance in which a foreleg was gnawed off during hibernation by a rat, and Frye (1994) reported digit necrosis in red-eared slider turtle, Trachemys scripta elegans.
Table 5
Dicephalic turtles
Species | Common name | Kind of duplication | References |
|---|---|---|---|
Cryptodira Cheloniidae | |||
Caretta caretta | Loggerhead | Dicephalic | Anon. (2002) |
Chelonia mydas | Green turtle | Duplication of head and forelimbs Second, atrophic head | Haft (1994) Coquelet (1983) |
Chelydridae | |||
Chelydra serpentina | Common snapping turtle | Dicephalic twins | Cederstrom (1931); Canella (1932); Campbell (1967); Feldman (1983); Sims (1989); Anon. (1995, 2001); Yntema (1970, 1971) |
Geoemydidae | |||
Chinemys reevesii | Reeve’s turtle | Dicephalic derodymus | Khosatzky (1991) Nakamura (1938) |
Emydidae | |||
Chrysemys picta | Northern painted turtle | Dicephalic derodymus duplication of head and forelimbs Siamese twins attached at the posterior ends | Barbour (1888, 1896a, b); Anon. (1889); Girard (1891–1892); Hildebrand (1938) Barbour (1896c); Derickson (1927); Townsend (1928) Bishop (1908) Hildebrand (1938) |
Chrysemys sp. | Painted trutle | Derodymus | Schmidt and Inger (1957); Bellairs (1981) |
Emys orbicularis | European pond turtle | Duplication anterior head | Epure and Pogorevici (1940) |
Emys sp. | European pond turtle | Dicephalic | Bateson (1894); Przibram (1909); Newman (1923) |
Graptemys pseudogeographica | False map terrapin | Dicephalic | Clement (1967); Sims (1989) |
Malaclemys sp. | Diamondback terrapin | Dicephalic, derodymus | Brogard (1987) |
Malaclemys terrapin | Diamondback terrapin | Dicephalic Derodymus | Anon. (1975); Wright (2005) Schmidt and Inger (1957); Frye (1991a) |
Malaclemys terrapin centrata | Carolina diamondback terrapin | Duplication of head and forelimbs, anakatamesodidymus | Hildebrand (1938) |
Mauremys leprosa | Mediterranean pond turtle | Dicephalic | Martins d’Alte (1937) |
Pseudemys sp. | River cooter turtle | Dicephalic | Anon. (2000) |
Pseudemys concinna floridana | Florida cooter | Dicephalic Siamese twins Plastron to plastron | Hildebrand (1938) Reichenbach-Klinke and Elkan (1965); Reichenbach-Klinke (1977) Hildebrand (1938) |
Pseudemys nelsoni | Florida redbelly turtle | Dicephalic Derodymus | Ippen (1985) Bellairs (1981); Ippen (1982) |
Terrapene carolina | Eastern box turtle | Twins Partial skull duplication | Cohen (1986) Edwards (1751) |
Terrapene carolina triunguis | Eastern box turtle | Twins | Crooks and Smith (1958); Cohen (1986) |
Terrapene sp. | Box turtle | Dicephalic | Mitchell (1994); Anon. (2006a) |
Trachemys scripta elegans | Red-eared slider | Dicephalic Derodymus
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |