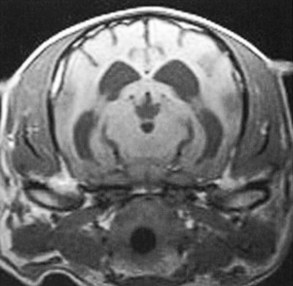
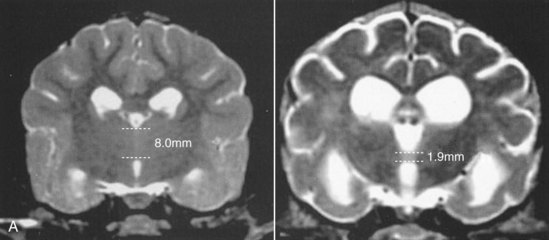
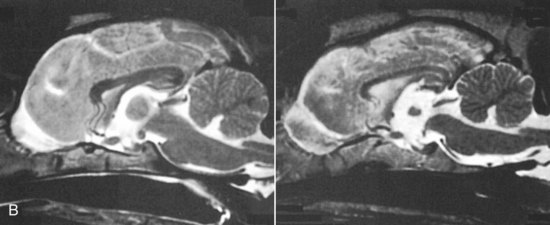
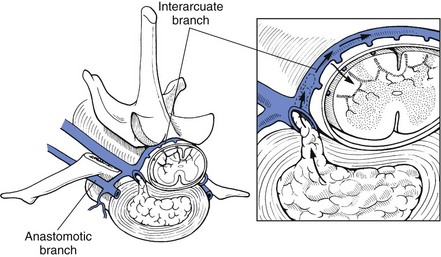
Chapter 43 Specific Diseases The only imaging modality of use for the diagnosis of CDS is MRI. Brain imaging of AD patients can be normal or may reveal brain atrophy, ventricular enlargement, and lesions in the medial temporal lobes of the cerebral cortex (Fig. 43-1). Age-related changes appreciated on MR imaging of the brain in CDS patients are primarily reflective of brain atrophy and include ventricular enlargement, widened and well-demarcated cerebral sulci, and diffuse and scattered areas of T2-hyperintensity in periventricular white matter. Although these are consistent findings associated with the aging brain, they may be found in older patients without evidence of CDS. The thickness of the interthalamic adhesion as measured on transaxial T1- and T2-weighted MR images was found to be significantly smaller in dogs with CDS compared with dogs without CDS (Fig. 43-2); an interthalamic adhesion thickness of 5 mm or less was found to be consistent with a diagnosis of CDS in dogs (Hasegawa et al, 2005). Laboratory findings are typically normal in dogs and cats with CDS, unless there is an age-related concurrent disorder (e.g., elevated BUN/creatinine from chronic renal disease). It is important to measure fasting/postprandial serum bile acids and/or blood ammonia to distinguish CDS from hepatic encephalopathy. Dogs with CDS should have normal serum bile acids and blood ammonia. Congenital portosystemic shunts are sometimes not diagnosed until the dog is older than 10 years old, and mild hepatic encephalopathy may mimic the findings expected in CDS (Mertens et al, 2010). There are numerous proposed therapeutic approaches to CDS, with variable evidence of efficacy in improving cognitive function and/or delaying progression of cognitive decline. The use of oral l-deprenyl (selegiline; Anipryl, Atapryl, Carbex, Eldepryl, Zelapar), an irreversible inhibitor of monoamine oxidase B (MAOB), has been purported to improve cognitive function and slow progression of the disease in the majority of dogs and cats with CDS. There is considerable variability in the degree of response achieved among patients, however. l-deprenyl is thought to exert its beneficial effects in the brain by restoring dopaminergic balance as well as enhancing catecholamine levels and decreasing levels of damaging free radical species. The dosage for dogs is 0.5 to 1 mg/kg every 24 hours. Cats are administered 0.5 mg/kg every 24 hours. Most patients will exhibit a positive response within the first month of therapy. Despite apparent positive responses of both canine and feline CDS patients to selegiline, there is evidence that this drug does not have a significant effect on cognitive function in these patients or in people with AD. The clinical efficacy studies supporting selegiline use in CDS are based primarily on owner response to questionnaires rather than on standardized comparative cognitive testing procedures of treated and untreated patients. Because selegiline may produce nonspecific low-level hyperactivity by increasing brain catecholamine levels, the “response” observed by owners may not truly be representative of improved cognitive ability. Selegiline is not considered an effective drug for human AD, because of variable responses and overall minimum improvement of cognitive function. The acetylcholinesterase inhibitor phenserine has exhibited efficacy in improving cognitive function in both dogs with CDS and humans with AD in clinical trials; to the author’s knowledge, this drug is not yet commercially available for dogs. Behavioral changes in CDS patients may be alleviated with the use of GABA-ergic drugs, such as gabapentin or pregabalin. Because inflammatory changes have been identified in the brains of CDS dogs, the use of anti-inflammatory drugs (e.g., carprofen) has also been proposed. Some naturally occurring phytochemicals (e.g., curcumin, resveratrol, green tea catechins) may hold some promise as treatment options for CDS. Oral S-adenosylmethionine (SAMe) has been shown to be effective in improving clinical signs of mental decline in CDS dogs in one study (Reme et al, 2008). Finally, a large number of complimentary therapies have been suggested for the treatment of CDS, with the primary goals of calming the patient, reducing anxiety, and normalizing the sleep-wake cycle. These include melatonin, valerian root, dog-appeasing pheromone (DAP), phosphatidylserine, ginkgo biloba, DHA (an omega 3 fatty acid), and various antioxidants and mitochondrial cofactors. The evidence for efficacy of these complimentary therapies is entirely anecdotal. There is convincing evidence that providing a diet fortified with antioxidants, mitochondrial cofactors, and essential fatty acids improves cognitive function and delays cognitive decline in dogs with CDS. This commercially available diet (Hills b/d) contains a mixture of fruits and vegetables, in addition to vitamins C and E, and mitochondrial cofactors (L-carnitine, DL-α-lipoic acid). Environmental enrichment, such as regular exercise and introduction of new toys, has also been demonstrated to improve cognitive function and delay cognitive decline in dogs with CDS. Progression of CDS appears to be more rapid in castrated versus intact male dogs, suggesting a potential role for hormone replacement therapy in this disease. Hasegawa, D, Yayoshi, N, Fujita, Y, et al. Measurement of interthalamic adhesion thickness as a criteria for brain atrophy in dogs with and without cognitive dysfunction (dementia). Vet Rad Ultrasound. 2005;46:452. Mertens, M, Willard, MW, Miller, M, Fossum, TW. Diagnosis of congenital portosystemic shunts in miniature schnauzers seven years old or older (1997-2006). J Amer Anim Hosp Assoc. 2010;46:235. Reme, CA, Dramard, V, Kern, L, et al. Effect of s-adenosylmethionine tablets on the reduction of age-related mental decline in dogs: a double-blinded, placebo-controlled trial. Vet Ther. 2008;9:69–82. Degenerative Myelopathy Degenerative myelopathy (DM) is a heritable (presumed autosomal recessive) progressive degeneration of axons and myelin in all funiculi of the spinal cord, primarily in the thoracolumbar region. Recent evidence (Awano et al, 2009) suggests that DM is the canine analog of human amyotrophic lateral sclerosis (ALS). A genetic mutation in the superoxide dismutase 1 (SOD 1) gene has been identified in dogs with DM; this mutation leads to intraneuronal accumulation of cytotoxic cytoplasmic aggregates that stain with anti-SOD 1 antibodies. Loss of axons with secondary demyelination has been demonstrated in both the central nervous system (CNS) and peripheral nervous system (PNS) of affected dogs. Dogs that are homozygous for the gene mutation are at risk for developing DM. This is a disorder that primarily affects older (≥8 years), large-breed dogs. The German Shepherd is by far the most common breed affected, but other dog breeds and one cat have been reported to be affected. Additionally, a form of DM has been described in Pembroke Welsh Corgis. There appears to be a female predominance for the disease in Corgis. A list of dog breeds believed to be predisposed to DM is provided in Box 43-1. Loss of pelvic limb proprioceptive ability (ataxia, toe-dragging) is noticed initially, followed by gradual loss of voluntary motor function. Spinal reflexes in the pelvic limbs are typically normal to hyperreflexive. Decreased to absent patellar reflexes are found in approximately 10% to 15% of patients, however, and may reflect selective damage to dorsal lumbar nerve roots in these dogs. Pelvic limb tremors may be present during weight support. Late in the disease process, urinary and/or fecal incontinence may develop. Dogs often develop disuse atrophy of the pelvic limb musculature over time (see the discussion under Prognosis). Spinal imaging (myelogram, MRI, CT) is typically normal, but some dogs may have concurrent mild type II disk lesions that are probably clinically insignificant. In one study of CT/myelography performed in dogs with degenerative myelopathy (Jones et al, 2005), a number of abnormalities were identified when compared with normal dogs, including vertebral canal stenosis, deformed spinal cord, small spinal cord, focal attenuation of the subarachnoid space, and paraspinal muscle atrophy. A definitive diagnosis of degenerative myelopathy is based on characteristic histopathologic lesions in the spinal cord at necropsy. Major differential diagnoses for DM are chronic type II disk protrusion and spinal neoplasia. Awano, T, Johnson, GS, Wade, C, et al. Genome-wide association analysis reveals a SOD 1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2009;106:2794. Jones, JC, Inzana, KD, Rossmeisl, JH. CT myelography of the thoracolumbar spine in 8 dogs with degenerative myelopathy. J Vet Sci. 2005;6:341. Ischemic/Vascular Disease There are multiple potential causes for brain infarcts, including systemic hypertension (primary/essential hypertension or secondary to underlying disease such as chronic renal failure, hyperadrenocorticism, or pheochromocytoma), cardiac disease, hypercoagulability, increased blood viscosity (e.g., polycythemia vera, multiple myeloma), intravascular neoplasia (e.g., lymphoma, hemangiosarcoma), infectious disease, and atherosclerosis (e.g., associated with hypothyroidism, diabetes mellitus, or hyperlipidemia). In people with strokes, an underlying cause is not identified in about 40% of cases; these infarcts are termed “cryptogenic.” The percentage of cryptogenic strokes in dogs is believed to be similar to that in people. In contrast to humans, atherosclerosis appears to be rarely associated with canine brain infarcts; when it does occur in dogs, it is most likely to be associated with hypothyroidism. Brain infarcts in dogs are typically nonhemorrhagic and are most common in the cerebellum, cerebrum, and thalamic/midbrain regions. Multifocal brain infarcts have been reported but are comparatively uncommon. Cerebellar and cerebral infarcts tend to be territorial, involving the territories of large arteries such as the rostral cerebellar artery and middle cerebral artery, respectively. These territorial infarcts tend to primarily involve the gray matter with variable levels of white matter involvement. Thalamic/midbrain infarcts tend to be smaller lacunar lesions involving the smaller perforating arteries of this brain region. In one report, over half of the dogs with brain infarcts had an underlying metabolic disorder that could potentially lead to thromboembolic disease, the most common being chronic renal disease and hyperadrenocorticism (Garosi et al, 2005). Systemic hypertension was documented in nearly 30% of those dogs whose blood pressure was documented. In another study of cerebellar infarcts in dogs, systemic hypertension was identified in over 40% of those patients in which blood pressure was measured (Darrin et al, 2006). In the author’s experience there is usually an underlying disease present that could potentially explain the presence of hypertension in these dogs, with chronic renal disease and hyperadrenocorticism being the most common disorders. Fibrocartilaginous embolic myelopathy is a common syndrome caused by the embolization of arterial and/or venous supply to an area of the spinal cord. The embolizing material has been identified as fibrocartilage and is believed to originate from the nucleus pulposus of the intervertebral disk. The mechanism or mechanisms by which this material reaches the spinal cord vasculature from the disk are unknown. Theories center either around venous entry of disk material (e.g., extrusion either directly into a venous sinus or venous system of vertebral bone marrow—a Schmorl’s node) with retrograde movement into the spinal arterial system (Fig. 43-3), or direct entry to the spinal cord arterial system (e.g., into normal surrounding vasculature or neovascularization over the annulus fibrosus associated with concomitant type II disk degeneration).
Nonsurgical Disorders of the Brain and Spine
Cognitive Dysfunction Syndrome
Diagnosis
Diagnostic Imaging
Laboratory Findings
Medical Management
References
General Considerations and Clinically Relevant Pathophysiology
Diagnosis
Signalment.
Physical Examination Findings
Diagnostic Imaging
Differential Diagnosis
References
General Considerations and Clinically Relevant Pathophysiology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nonsurgical Disorders of the Brain and Spine