Fig. 1.
Spinal cord transection. The vertebral column is gently tilted up with forceps allowing microscissors to cut intervertebral ligaments and to be inserted intervertebrally for a transection of the spinal cord.
2.3 Recovery and Post-op
Anesthesia is interrupted and mice are placed in a large cage with a heating pad put underneath. It is critically important to use only minimal heating intensity (35°C) to avoid rapid dehydration, heat shock, and death during the recovery period. Generally, the animals will recover completely within 15 min although we normally leave them on the heating pad overnight with free access to food and water. The recovery procedure is critical to ensure a high percentage of survival post-surgery (typically around 95% in our laboratory). The following day, the animals are replaced in their initial cage with their initial mates to reduce potential aggressions and fights.
Postoperative care is provided a few hours after surgery as well as every day for the next 4 days. It includes injections of lactate-Ringer’s solution (2 × 1 ml/day, s.c.), buprenorphine (2 × 0.1 mg/kg/day, s.c.), and Baytril (5 mg/kg/day). Bladders are also manually emptied twice a day until a spontaneous return of some micturition reflexes. For voiding, the bladder has to be delicately pressed between the thumb (side of the bladder) and two fingers (e.g., the index and one other finger placed on the other side of the bladder). This maneuver requires time and experience. In male mice, it is specifically challenging since, in addition, penises have to be continuously maintained against a paper towel to improve successful voiding (i.e., seems to contribute to the expulsion of urine outside the urinary tract probably by capillary action). The belly and sexual organs are also cleaned daily using paper towels and chlorhexidine gluconate solution (0.05% v/v) to prevent urinary tract infection. Normally, with this procedure, mice that survive the first 24 h, will remain relatively healthy for a long period of time (i.e., several months). Finally, Michel suture clips are removed after 10 or 14 days post-surgery. Cages have to be cleaned regularly (if possible changing cages every 3 or 4 days) and mice have to be washed, as mentioned above, on a daily basis to prevent urinary tract infection.
All in all, once anesthetized, this surgical procedure takes no longer than 5 min whereas another 10 or 15 min is typically required for animals to recover from anesthesia. Reasons for using a no-laminectomy approach are multiples. Firstly, since we aim at obtaining a complete transection, a minimally invasive approach such as this one (i.e., inserting microscissor between two vertebrae) can be performed with ease. Obviously, this would be more complicated in studies where an incomplete spinal cord lesion is seeked (e.g., contused or crushed spinal cord). Secondly, avoiding the laminectomy part saves time, reduces periods of anesthesia, and, thus, probably contributes to increase survival rate, recovery, and health post-surgery. Thirdly, a no-laminectomy approach prevents many subsequent problems generally associated with laminectomies (e.g., which are known to reduce vertebral structure integrity and to induce lordosis, epidural fibrosis, altered biomechanics, pain-related behaviors, etc.).
3 Spinal Cord and Systemic Adaptations
Within a few days to a few weeks post-surgery, spinal cord-transected mice undergo a wide variety of adaptations at the spinal cord level (sublesionally) as well as the system level (e.g., muscles, bones, hormones, blood, etc.). Many of these adaptations have been summarized elsewhere (21–24). In brief, immediately following surgery, a complete and irreversible loss of sensory and voluntary motor function is found in these animals. A 1–2-mm-wide scar is found at the site of transaction which is responsible for a complete interruption of descending and ascending signals between the lumbosacral segments and the brain (Fig. 2).
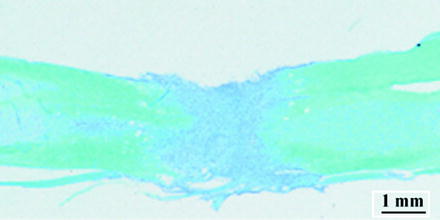

Fig. 2.
Spinal cord histology. Luxol blue and cresyl violet staining of a longitudinal section of the spinal cord from a spinal cord-transected mouse 1 week post-surgery.
3.1 Spinal Cord Adaptations Distally from Epicenter (Sublesionally)
Although, in other animal models (e.g., incompletely injured following contusion or crush), several cellular events have been reported to occur at the site of injury (epicenter) (25–30), comparable processes have not been characterized in spinal cord-transected models probably because it is irrelevant—i.e., regeneration after a complete transection has never been reported in mammals.
In turn, several cellular events have been found to occur distally from the site of injury (27). Recent findings from this animal model and other comparable models have shown that neurons located caudally from the epicenter (so-called sublesional neurons) remain alive and functionally viable (31–35). This said, sublesional neurons were reported also to undergo a number of plasticity and adaptive events within a few days to a few months post-trauma (22, 32, 34, 35). The next sections provide a list of the main sublesional adaptive changes reported in animal models of SCI and, specifically, in this no-laminectomy spinal cord-transected mouse model.
3.1.1 Immediate Early Genes and Other Cytokines
Immediate early genes (IEGs) constitute a large family of genes well-known as early regulators of cell growth, differentiation signals, learning, and memory (28, 30, 36–38). Most recently, some IEGs were found also to be modulated post-trauma in sublesional spinal cord areas (27). For instance, Landry and colleagues have reported in low-thoracic spinal cord-transected mice that c-fos and nor-1 were respectively increased and decreased within a few days in the segments L1–L2, specifically in the dorsal horn and intermediate zone areas (39). In cervical or thoracic spinal cord-transected rats, increased fos-immunoreactive levels were also found as early as at 2.5 h post-trauma in lumbar laminae I–IV, VII, VIII, and X. Changes in the lumbar spinal cord of rostrally transected animals is of special interest since some of these segments (e.g., L1–L2 in mice) were shown to contain critical central pattern generator (CPG) elements (40). Given that IEGs are better known for their role in CNS development and plasticity, spontaneous changes of IEG expression (i.e., specifically c-fos and nor-1) in L1–L2 segments may be considered as among the first sublesional cellular events associated with altered cellular functions and properties post-SCI. This said, some of these changes may be associated also with other phenomena than plasticity or reorganization of spinal motor and locomotor networks. For instance, c-fos and nor-1 were used as markers in experimental models of pain and transient global ischemia suggesting a role in several functions (36, 38). In other studies, c-fos transcripts were found to drastically increase for up to 6 h near the site of lesion and to augment later in some distally located neurons (28). Such a regional- and time-dependent increase in IEG expression, which was found also in this study (39), points toward distinct roles for IEGs in cell death at the injury site versus in reorganization and plasticity at a distance from the epicenter.
Numerous regulatory changes are likely to be subsequently activated after altered IEG expression. These may include Tumor Necrosis Factor-alpha (TNF-alpha), preprodynorphin, and nitric oxide synthase (NOS), C1qb, Galectin-3 and p22(phox) which expression levels were found to increase in lumbar spinal cord segments for several days soon after a low-thoracic transection or injury in rats (29).
3.1.2 Transmembranal Receptors
Other key elements including transmembranal receptors may be considered as good candidates for plasticity and reorganization of motor and locomotor networks located sublesionally following a spinal cord-transection (and probably to some extent also after partial injuries). For instance, glycine receptors and GAD-67 (enzyme in GABA synthesis) were found displaying increased expression levels in lumbar spinal cord segments 3 months after a low-thoracic transection (32, 41). Increased 5-HT1A/7 receptor expression was detected using autoradiography with (3H) 8-OH-DPAT in lumbar spinal cord segments of spinal cord-transected cats (42). In spinal cord-transected mice, we found using in situ hybridization increased 5-HT1A mRNA levels in L1–L2 segments in 5-HT7-deficient mice compared with wild-types (43). This was interpreted as evidence suggesting that even greater changes may occur post-trauma in the absence of functionally closely-related genes. Results in mice revealed also increased 5-HT2A mRNA levels in lumbar segments (laminae VII, VIII, and IX) several days after a low-thoracic transaction (22).
3.1.3 Neurotrophic Factors
Although not specifically examined in spinal cord-transected mice, factors well-recognized for their role in development and plasticity have also been found to be modulated post-transection. Indeed, NGF, BDNF, and NT-3 were found displaying up-regulated levels of expression in sublesional areas of low-thoracic-transected rats (32). In contrast, BDNF and NT-3 as well as synapsin-1 were found to display lower levels of expression in the lumbar cord of low-thoracic-transected rats that were also deafferented which may suggest that these decreasing levels were associated specifically with low levels of muscle reflex activity due to deafferentation (22, 32).
3.2 Systemic Adaptations
A plethora of adaptive changes associated with several systems has been described in the spinal cord-transected mouse model. Interesting, most of these changes correspond with comparable adaptations found specifically in severe SCI cases (see Chap. 1).
3.2.1 Body Weight and Muscular System
We found that the body weight of this animal model significantly decreases soon after transection. A rapid loss typically ranging between 20 and 25% of initial body weight was found after only 1 week post-surgery (21). No significant return to normal levels was reported after 4 weeks although a moderate augmentation of approximately 1 g/week was found. Post-mortem measurements of individual limb masses revealed greater losses in hindlimbs (−28%) than in forelimbs (−21%). These relatively rapid adaptive changes were somewhat comparable to those generally reported soon after trauma in patients. Reasons underlying this initial weight loss are not fully understood but may involve reduced physical activity (or complete immobility), metabolic changes and hormonal deregulation. In turn, after a few years, this initial weight loss is often transformed into a weight gain leading to overweight and obesity problems in patients.
As mentioned above, hindlimbs are specifically affected in spinal cord-transected paraplegic animals. Results from our laboratory showed that part of this weight loss is due to a specific decrease in muscle mass. For instance, measurements from soleus muscles (ankle extensor) revealed a 32% decrease in mass at 1 week post-transection in mice (21). This decrease in mass was found to correspond to a proportional decrease in strength (absolute maximal tetanic force). Interestingly, in 2-month-spinal cord-transected mice, slow-twitch fibers (type I) from the soleus were found to progressively acquire some of the biochemical and contractile properties of fast-twitch fibers (fiber-type conversion). Again, evidence of comparable changes has been found in chronic SCI patients (2–4, 8).
3.2.2 Skeletal System
SCI is also associated with a rapidly increasing risk of fracture in patients. In fact, nearly all SCI individuals experience a significant loss of bone mineral tissue (up to 30% in the femora) within only a few months (7, 10). Comparable changes are found in spinal cord-transected mice, where histomorphometric data from our laboratory revealed a drastic decrease in trabecular bone volume (−22%), thickness (−11%), and number (−15%) within only 1 month post-surgery (44).
After 2 months, densitometric measurements from mouse femoral bones using dual-energy X-ray absorptiometry reported a significant decrease (approximately 15% lower) of bone mineral density (BMD) and bone mineral content (BMC). There is evidence from mice suggesting that rapid bone loss in such conditions may involve a sharp decrease of osteoblastic activity and a rapid increase of osteoclastic activity (i.e., low osteocalcin and high acid phosphatase levels) (45).
3.2.3 Vascular System
Soon after SCI, there is a well-known risk of developing deep venous thrombosis (DVT) in the lower limbs and, hence, cardiovascular and pulmonary complications in patients (1). However, the specific mechanism underlying DVT formation following SCI remains poorly understood. Our model may be used to study this specific problem. We found using in vivo confocal microscopy that deep vein morphology is largely changed as rapidly as after 1 week post-spinal cord-transection (46). The femoral and saphenous veins were clearly found to largely increase (>1.5-fold) in diameter which may suggest that DVT formation after SCI is associated with venous stasis (reduced blood flow) due to blood vessel enlargement.
3.2.4 Immune, Blood, and Bone Marrow Systems
Immune deficiencies may lead to life-threatening complications after SCI. To examine possible factors that may contribute to this pathological condition, we used an automatic blood analyzer to characterize changes in red and white blood cell counts in spinal cord-transected mice. The results showed that total leukocyte numbers decreased by 35% at 1 week post-surgery and remained low subsequently (47). Specifically, lymphocyte numbers were reduced by as much as 53% at 1 week post-transection whereas monocytes and neutrophils generally remained unchanged. In turn, eosinophil counts were found to gradually decrease by 81% after 4 weeks. Analyses from bone marrow samples revealed similar changes.
Blood lipid profiles examined with clinical chemistry analyzer revealed decreased concentrations of cholesterols (−25%), triglycerides (−45%), low-density lipoproteins (LDL, −55%), high-density lipoproteins (HDL, −14%) but not platelets. Comparable data (e.g., low LDL-triglyceride levels) have been reported in acute SCI patients (47). Erythrocyte, platelet, hemoglobin, and hematocrit levels were either unchanged or moderately decreased (mild anemia) at least for 1 month post-transection.
3.2.5 Anabolic Hormone Systems
Serum levels of testosterone, GH, DHEA, PTH, and insulin were studied using ELISA in control and spinal cord-transected mice. We found early transient changes in testosterone (−40% at 1 week) and GH (threefold increase) levels during the first 2 weeks post-transection (47). Other hormones undergo apparently sustained changes such as DHEA (−75%), PTH (−75%), and insulin levels (−84.5% at 1 week) which were consistently reduced throughout the time period studied (1 month post-surgery). Comparable changes have been found in humans since shortly after trauma, men were found displaying decreased testosterone levels for several weeks whereas increased GH and decreased PTH levels were reported in late chronic SCI patients (6). It remains unclear what role these hormonal changes may play in health degradation post-SCI. However, results in mice suggest that some of these changes may participate to immune deficiencies since high temporal correlations were found between specific anabolic hormones and immune cell count changes.
The many anatomic, systemic, and metabolic changes or adaptations reported here suggest that this mouse model may be useful to study SCI-related secondary complications typically found also in severely SCI patients. These detailed quantitative data measured in mice are critically important to properly assess and compare in subsequent studies the potential effects of innovative drug treatment candidates to prevent or reduce these adaptations and related health problems in chronic SCI individuals.
4 Specific Approaches Developed to Measure Drug Treatment-induced Recovery in Spinal Cord-transected Mice
As mentioned earlier, several adaptations may occur distally from the epicenter and, as reported in Chap. 2, several spinal cord circuits may be affected given their known localization in segments of spinal cord generally spared (lumbar and sacral segments) after SCI (most patients are injured at the cervical or thoracic level). Although, clear evidence exists that neuronal circuits controlling locomotion, micturition, or ejaculation (48) remain functional even several months after a spinal cord transection in mice, behavioral assays specifically developed to study their activity following drug administration remain largely lacking (11, 49, 50).
Most other vital functions remain relatively unaffected (cardiac, respiratory, feeding, voiding functions are not significantly impaired) after a low-thoracic spinal cord transection but sublesional circuits involved in locomotion, micturition, and ejaculation become either quiescent or improperly active (i.e., often reflexively) (48).
4.1 Locomotion
4.1.1 Description of ACOS
In order to examine pharmacological approaches aimed to re-activate properly each of these networks, corresponding quantitative assays had to be developed. For locomotor CPG reactivation, we created a novel quantitative assay called ACOS which stands for Average Combined Score (51). In brief, it allows assessing separately locomotor-like movements (LM) and non-locomotor movements (NLM) in the hindlimbs of spinal cord-transected mice. LM and NLM incidence, frequency, and amplitude are normally assessed during 4-min (video-recorded or not) prior to drug administration and after drug injection. A representative single score can be conveniently obtained for each animal by combining arithmetically the assessed values mentioned above as follows: ACOS = (NLM + (2 × LM)) × amplitude). One LM is defined as an entire step-like cycle consisting of an extension phase followed by a flexion phase occurring in both hindlimbs consecutively (i.e., bilaterally alternated or out-of-phase relation). Weight-bearing is not assessed and does not need to be expressed for LMs to be considered as such. One NLM is defined as one non-bilaterally coordinated movement (i.e., not followed by a flexion-extension on the other side). They include unilateral movements, jerks, brief sequences of fast-paw shaking (typically lasting 1–2 s/episode and counted as one NLM), twitches, and kicks. Amplitude is assessed simply by assigning one of three values; 0—if no movement is observed; 1—if the amplitude of most movements is less than half the range of motion of normal steps; 2—if the amplitude of most movements is at least more than half the range of motion of normal steps. Note that amplitude is assessed for LM and NLM indistinctively. Incidence is not measured per se but is obtained when averaging ACOS scores from all mice of a group. It corresponds to the number of mice (out of all mice tested in a group) in which NLMs or LMs were observed. Plantar foot placement and body-weight support may be reported as either present or not although they do not contribute to the equation used to obtain ACOS (i.e., in general, spinal cord-transected mice cannot be made to display weight-bearing stepping without drug or even with most drugs (for details, see Sect. 5.1)). Note that, in the equation, LM is multiplied by a factor of “2” for a very simple reason—even if the experimenter counts each LM as a one movement, an LM is a bilaterally alternating movement which as such is in fact constituted of two movements (one in each hindlimb). Consequently, LM values are multiplied by a factor of “2” whereas NLMs are not (typically unilateral movements) (34, 51).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


