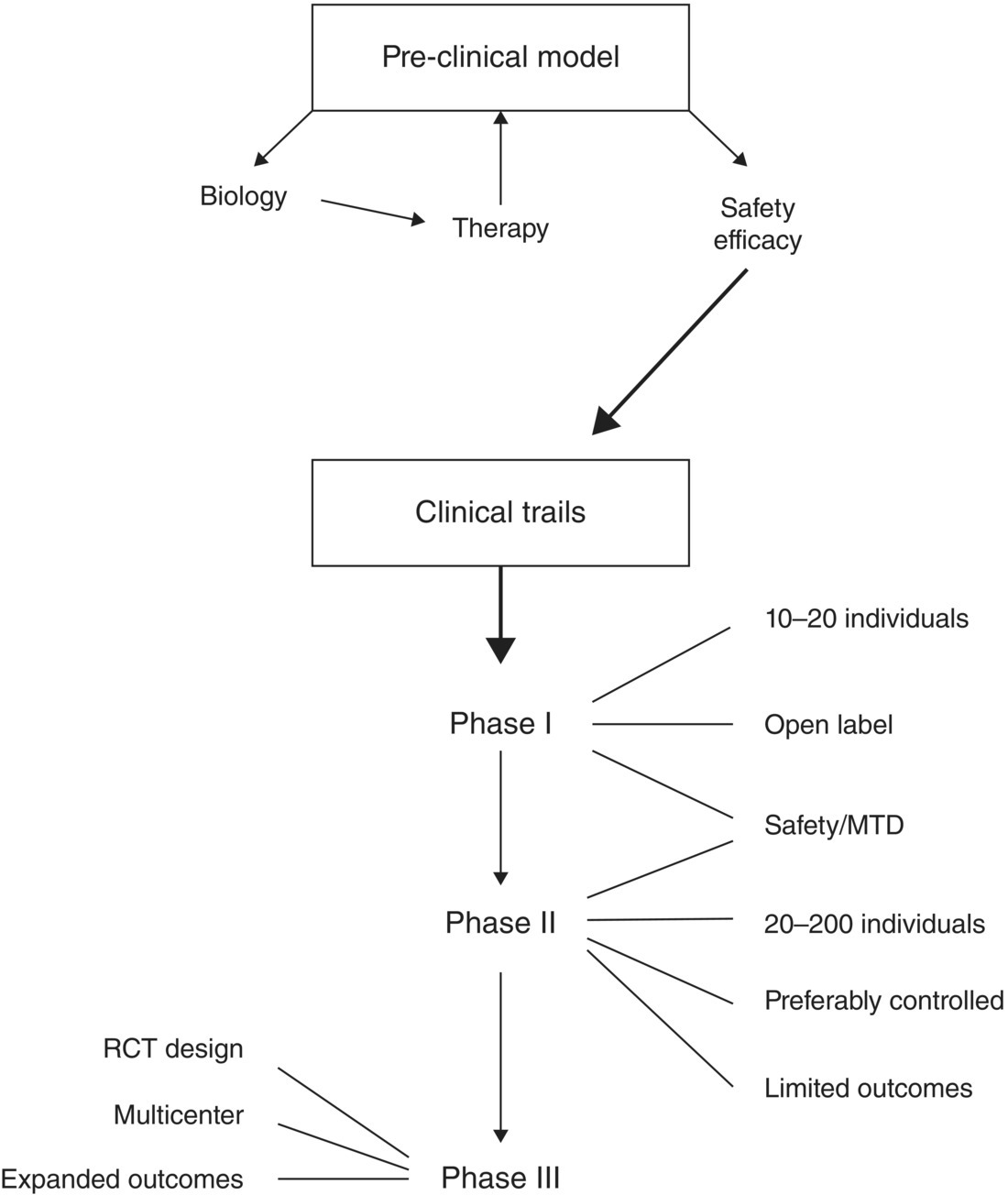
25 Jonathan M. Levine Medical treatments for spinal cord injury (SCI) are typically classified as neuroprotective, regenerative, or plasticity inducing [1]. Neuroprotective strategies attempt to mitigate acute secondary SCI. Regenerative strategies facilitate the remyelination and regrowth of axons through injured areas as well as the development of new cellular constituents. Plasticity-inducing treatments focus on altering function or connectivity of existing parenchyma in order to improve outcomes. In some instances, therapeutics may have mechanisms of action that encompass more than one of these three categories. To date, a limited number of neuroprotective therapies have been systematically evaluated in dogs with acute SCI resulting from intervertebral disc herniation (IVDH). This chapter will review modern, accepted means of developing and testing novel therapeutics, define commonly used outcome matrices, and review published clinical trial data. This chapter will not address glucocorticoids, which are among the mostly commonly used and controversial neuroprotective agents, as these are discussed in detail in another chapter (see Chapter 23). Typically, potential treatments for SCI are first investigated in animal injury models. The advantages of using preclinical SCI models are that these systems are inexpensive, are easily reproducible, induce relatively homogeneous parenchymal changes, permit histologic assessment of injury at established time points, and have well-characterized functional outcomes [2]. The majority of rodent injury models induce contusive SCI by use of an impactor that rapidly deforms the plane of the spinal cord in a reproducible manner [3]. Clip and crush models are also used by some investigators. Promising neuroprotective agents are identified based on pathomechanisms or targets that have been characterized in rodent models. These drugs are then delivered in model systems to determine safety and efficacy. The assessment of efficacy is typically performed using a placebo group, with or without investigator blinding; outcomes such as histologic lesion volume, gait scores, or electrophysiological responses are assessed. There are weaknesses in this approach. For example, rodent models do not mimic lesion heterogeneity, all primary injury mechanisms, and variation in timing seen in naturally occurring disease [4, 5]. Additionally, many preclinical rodent studies are not externally validated. Recent evidence from the National Institutes of Health “Facilities of Research—Spinal Cord Injury” project suggests that when independent replication of rodent studies is performed previously detected outcome associations often are not present or significantly smaller in magnitude. This unfortunate reality has been borne out with neuroprotectives such as erythropoietin, Nogo-66 antagonists, and minocycline [6–8]. Drugs that show promise in animal models—preferably in multiple studies from independent laboratories—are then taken to clinical trials. When a drug is introduced into clinical trials in animals or humans with naturally occurring SCI, this is done in phases. Phase I studies usually involve a small number of subjects (10–20) with the primary goals being establishing dose and safety data. Phase II studies (20–200 subjects) continue to evaluate safety at a preset dose and also gather preliminary data regarding efficacy over a limited number of outcome measures. Phase II studies may be controlled or uncontrolled, may utilize blinding, and always are performed at a single institution. Phase III investigations are performed following encouraging phase II data; they are randomized, double-blinded, placebo-controlled studies that use a multi-institutional population. Meta-analysis is a technique that allows data from several similarly constructed trials to be combined to address issues such as efficacy and toxicity. In human medicine, this type of synthesis is often performed to help set standards of care. Figure 25.1 summarizes the “pipeline” algorithm for studying prospective therapeutic agents. Figure 25.1 Schematic illustrating the typical “pipeline” for development of novel medical therapeutics. The breadth and number of neuroprotective drugs currently under investigation in animal models is staggering [9]. Some of the more thoroughly evaluated therapeutics include minocycline, polyethylene glycol (PEG), hypothermia, magnesium, erythropoietin, nonsteroidal anti-inflammatory drugs, anti-CD11d antibodies, estrogen, progesterone, matrix metalloproteinase antagonists, and various antioxidants. In most instances, results in models have been conflicting, divergent in their magnitude, not replicated independently, or available in only one species/system.
Neuroprotective Treatmentsfor Acute Spinal Cord Injury Associated with Intervertebral Disc Herniation
Introduction
Animal models: The first step
Clinical trials
Neuroprotectives studied in models
Outcomes in dogs with IVDH
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


