Fig. 1.
Tests from the modified SHIRPA screen. Force touch assesses the reactivity of the mice to a touch on the back from above (a). The use of a beam or a grid can be used to determine the grip strength of the mouse (b) when pulled gently by the tail on the horizontal plane (picture taken from above), and the same position is the starting point for the wire climb task (c). Negative geotaxis assesses the ability of the mouse to rotate through 180° from a head down position (d). In this instance, a cage lid was used as a climbing mesh.
For Set 3, the assessment of the mice is made either on or above the arena whilst being handled by tail grip between the thumb and forefinger. Trunk curl and limb clasping are scored as either absent (0) or present (1). Visual placing determines whether the mice display the normal forelimb reaching reflex when being lowered by the tail on to a flat surface and is scored from absent (0) to early extension of the forelimb from 25 cm away or more (4). Grip strength (Fig. 1b) is measured by allowing the mouse to grip a metal grid (12-mm mesh) that covers a section of the arena top, and is assessed as being absent (0) through to unusually strong (4). Toe pinch is the gentle compression of the mid digit of a hind foot with a pair of forceps and measures the pulling away reflex. Toe pinch is measured on a 5-point scale from “no reflex” (0) to “strong repeated pulling motion” (4). The final manipulation with Set 3 uses a horizontal wire (∼3 mm diameter) to determine the ability of the animal to grip with its hindlimbs (Fig. 1b, c). The mouse is lowered onto the wire whilst holding the tail, and then with constant but gentle force is manipulated into the horizontal position whilst gripping the wire with the forepaws. The mouse is then released from the grip and is scored as “falls immediately” (0) to “active grip with hindlegs” (4), for its ability to grip with the hindlegs.
The measures that comprise the complete set of tests for Set 4, are less overtly neurological in nature and record parameters of general health such as skin colour and heart rate. Nevertheless, righting reflex, contact righting reflex and negative geotaxis are all measured. Righting reflex examines the ability of the mouse to land on its feet after being flicked in the air by the tail such that it undergoes a backward somersault. The height of the manoeuvre should be the minimum required to complete the assessment. Prior to flipping the mouse in the air, the mouse should be placed on its back to determine whether it is able to right itself under normal circumstances, with a failure to do so producing a score of 0. In this instance, the mouse should not be flipped. If the mouse has an intact righting reflex and is subsequently flipped, scoring is dependent on whether it lands on its back, side or feet resulting in respective scores of 1, 2 or 3. For the contact righting reflex, the mouse is placed in a narrow horizontally positioned perspex tube (30 cm × 3 cm diameter), which allows the animal to have contact with a surface to aid its recovery to a normal position. The tube is rotated through 180° such that the mouse is upside down. The contact righting reflex is either absent (0) or present (1). Finally, negative geotaxis (Fig. 1d) refers to the ability of the mouse to turn on a vertically positioned grid from being in the head down position to the head up position by rotating the body through 180°. Some mice may not be able to maintain their position on the grid at all (scored as 0), or may freeze for the duration of the 30 s test (1), move slightly but not turn (2), turn and freeze (3) or turn and climb the grid (4).
The scores from the SHIRPA allow the researcher to produce an overview of a mouse line, with the greatest value of this approach being that it provides the researcher with a clear indication towards the nature of the deficits, which may then be assessed in greater detail with additional tests. However, the data from the SHIRPA can also stand alone as a valid assessment of neurological deficits in a mouse line.
2.3 Notes
The modified SHIRPA provides a rapid assessment of any underlying deficits that a mouse model may have, but the cost of the observational rating scale approach is that it lacks sensitivity and the scientific rigour associated with more objective approaches. Whereas the mice do not require and indeed should not be trained on any aspect of the screen, it is crucial that the research scientist is trained to produce consistent scores between the mice on each aspect of the assessment procedure. The lack of objectivity is more problematic when more than a single individual scientist is assessing the animals, which then requires a high degree of rating reliability between the individuals. This can be difficult to achieve and can drift with time requiring the retraining of staff.
Whilst there are several tests that comprise the SHIRPA, it is not necessary to run them all and specific probes of function can be extracted to suit ones needs. Likewise, variations in the design of the apparatus are also acceptable, but it should be considered that the SHIRPA is designed to be a standardised test procedure, consequently modifications of the test equipment could exclude the results from being regarded as a SHIRPA screen. Nevertheless, most laboratories are likely to have similar but not exact pieces of equipment that would suffice for the tests required, and as such would provide valuable readouts without additional expense.
As the SHIRPA uses rating scales, the statistical approach for the analyses has to be non-parametric in nature, and therefore has less statistical power than tests that generate interval or ratio data sets. As a consequence, the use of statistical tests other than the standard analysis of variance is required, such as the Mann–Whitney U-test or Kruskal–Wallis non-parametric analysis of variance, where the computations are based on median values rather than means. This general lack of sensitivity may account for the reported discrepancies in the literature, where conventional tests of motor function return significant differences between synphilin-1 parkinsonian mice and their wildtype littermates that were not detected by the modified SHIRPA (9).
3 The Rotarod (Rotorod)
3.1 Introduction
The rotarod test was originally developed in the 1950s (10), specifically for the evaluation of neurological deficits in rats and mice and is now one of the most widely used tests of motor function in the mouse. The test measures motor coordination by assessing how long the animals are able to remain on a horizontal, rotating beam. The original design of the apparatus employed a number of fixed speed rotations, but an accelerating beam method (11), where a small number of runs on a beam that increases in speed from 0 rpm for a predefined time, is now more commonly used. Whilst the apparatuses may be identical in design for the two methods, the tests measure different aspects of motor coordination as the accelerating version of the task requires the mouse to continually adapt its gait in response to the changing speed of the rod. Consequently, the tests may be differentially sensitive to the same neurological insult, or be able to differentiate between subtly different lesions caused by the administration of the same toxin (12). In mice, the rotarod has been demonstrated to be a sensitive method of assessing disease progression in acute or longitudinal studies of neurodegeneration, for example in genetically- or chemical-induced neurodegeneration in mouse models of HD, PD, cerebellar ataxia, Niemann-Pick disease and amyotrophic lateral sclerosis (13–28), but can also discriminate lesioned from non-lesioned mice (29, 30), and between different intact mouse lines (31–33). It is a simple and rapid test to execute, promoting a higher throughput of animals and thus increasing readout sensitivity further, and provides instant behavioural data that require little expertise to interpret.
3.2 Methods
Rotarod apparatuses can be made in-house, but more commonly are acquired commercially. One of the most commonly used commercially available mouse rotarod apparatuses is the Ugo Basile apparatus (Fig. 2) that comes with a 3 cm diameter rod that has ∼1 mm horizontal grooves on the surface, which are designed to provide the mouse with better grip whilst running the task. The rod length is 30 cm, sub-divided with four partitions to create separate berths such that five mice may be run simultaneously. Below the beam in each of the five berths is a trip switch linked to a timer. The switch is raised slightly at the onset of each trial which initiates a timer for the specific birth, which is subsequently stopped when the mouse falls from the beam and lands on the switch, forcing it into the inactive position.
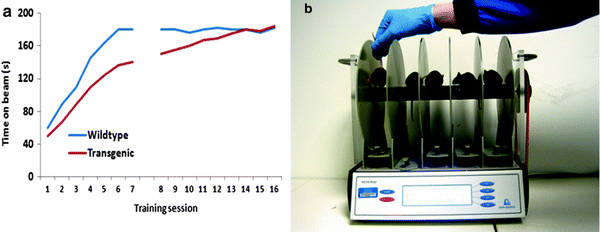

Fig. 2.
Extensive training schedules that permit the mice to reach asymptotic performance are essential for optimising behavioural readouts and for valid interpretation of the data (a). Mice are sensitive to distractions when on the rotarod. Simple precautions such as loading the machine in an appropriate manner, avoiding the casting of a shadow or placing a moving arm above the mice (b), can help prevent erroneous results from being returned.
Commercial rotarod apparatuses have several electronically controlled settings that permit the researcher to produce customised trial protocols. Trial duration, rod speed and rotation direction can all be specified, as can the rate of acceleration of the rod for accelerating rod trials. The ability to customise the trials can greatly increase the sensitivity of the test, in contrast to trials on in-house built equipment that may only function at a single set speed.
The key to providing consistently high quality, low variance data sets, is in the execution of an extensive training regime that permits the animals to attain stable asymptotic performance prior to testing. The amount of training required varies between mouse lines and disease models, but typically will take in excess of eight trials beyond the preliminary beam exposure, to assure that peak performance has been attained. For the initial exposure to the beam, the mouse is placed on the rod turning at a low speed, for example 5 rotations per minute (rpm), where it is permitted to remain for 1 min. In this period, the mouse will explore the apparatus whilst maintaining its balance on the beam. If the animal falls or jumps from the beam, it must be replaced immediately. The beam is then accelerated with the mouse in situ up to 10 rpm, where the pace of the beam is maintained for a further 1 min. At this speed, the mouse becomes more focused on remaining on the beam. After the 1 min has elapsed, subsequent increases in speed should take into account the specific features of the mouse lines being tested, for example age and level of disability in the cohort. For normal C57BLK/6j mice or mice at an age without an overt phenotype, 3 min at speeds of over 30 rpm are advisable. On achieving a level of competence on this initial exposure to the beam, the proper training trials can begin. These training trials are defined by whether the accelerating or stepped version of the task is to be used. Training for the stepped trials does not require the animal to be trained on each of the steps that are to be used in the test, but ideally would require the animal to perform consistently at the highest speed they are able to attain, and for the test duration that the experimenter wishes to use when collecting data. One additional option here is to train the animals on each of the speeds that are to be used in the study and collect the acquisition data as a measure of motor learning, but this slows the training process. For the accelerating version of the test, the animal is placed on the rod rotating at the lowest speed, and the trip switch is set to record task acquisition (this is optional, but provides a readout of motor learning), with the training trial starting with the onset of the acceleration procedure. If the mouse falls from the beam, it can be replaced as long as the beam is not travelling too quickly for the safe placement of the mouse. Replacing the animal is also not possible if the acquisition data are being collected for either of the test protocols. These extensive training schedules ultimately provide a high baseline providing the most sensitive assessment of performance degradation as the disease develops, for example.
The aim of this training regime then, is to allow the mouse to learn the operation of the task. Whether the mouse is running on the accelerated or the stepped protocol, the test trials for each animal are terminated when the animal falls from the beam landing on and triggering, a trip switch situated directly below each of the five berths of the rod. When triggered, the trip switch stops the timer associated with that particular berth, providing an accurate measure of how long the animal was able to remain on the rod. For the test procedure, it is advisable to use more than a single run per test session to produce consistent results. Typically in our laboratory, three runs per session are used for the accelerating rotarod with the first run being a practise run followed by the test runs at 15-min intervals. The data for the test runs can be averaged for each animal to reduce the effect of outliers. To avoid the effects of fatigue, at least 15 min should be allowed between the runs for each animal. On the Ugo Basile accelerating rotarod, the beam starts at 0 rpm and accelerates to 40 rpm over a 5-min period. For the stepped speed protocol, any number of speeds can be selected within this range and run for any duration, with the trade-off being the increased number of steps taking longer to run for each mouse which can make for prolonged testing sessions. Typically, five speeds of 1 min duration would produce good sensitivity.
3.3 Notes
One of the major issues with the rotarod is the length or number of training sessions required to attain a level of performance that represents asymptotic motor performance. Typically in the literature, researchers fail to train their animals or give them minimal training prior to data collection. This raises questions regarding the validity of their interpretations of the data, do they (transgenic or lesioned mice for example) have a motor deficit as reported, or a motor learning deficit? In the latter case, they may be able to perform the task as competently as their wildtype or sham lesioned counterparts, but due to the lack of training are not permitted the opportunity to express this, as they fail to acquire the task as rapidly as the comparator group (Fig. 2a). With continued training, the transgenic or lesioned mice may be able to achieve their asymptotic level of performance that may not differ from that of their wildtype littermates. Each cohort of mice or disease model must undergo a training regime based on their own abilities, and the researcher should refrain from attempting to use a standardised protocol across different groups.
Although the rotarod is a simple and effective measure of motor function, performance on the task can be affected by a number of factors. As with many behavioural tests, distracting stimuli within the environment are able to cause the mice to fall from the beam, consequently the test must be run in a suitable testing room that is quiet and free from distracting stimuli. Potentially, the greatest distracting influence on the performance of the mice is the research scientist, particularly when placing new mice on the rod when other mice have just started their test. The experimenter should ensure that if they are right handed they load the five berths of the rotarod from left to right (vice versa for left handed people), thereby insuring that mice that have already started the test are not exposed to the experimenters’ hand/arm moving around directly above their heads (Fig. 2b). Whilst at the onset of trials, the trials can be restarted if it is clear that the performance deficit was due to extraneous causes; however, this may cause additional problems if the animal that was upset by the distraction has to be placed back on to the rod, whilst other mice are running. Often it is advisable to utilise four or less of the berths rather than all five if the mice being used are particularly sensitive to distractions, and it should be recognised that the more difficult the task requirement, the more susceptible the mice will be to falling from the beam.
In some instances, the mice will lose their footing on the beam, but fail to fall from it, instead clinging to the beam surface and rotating with it. In this instance, the trial should still be classed as a fail as the mouse has lost its footing and is simply preventing itself from falling from the rod. Where possible, the time should be recorded as soon as this event takes place. In order to prevent this from happening, the ridged surface of the rod can be covered with a smoother material that makes it difficult for the mice to grip when they lose their footing. In our laboratory, we use rubber from a bicycle innertube which is not slippery to the touch even when wet, but allows the animals to fall and trip the timer when they lose their footing.
Weight differences between the control group and the experimental group, whether drug-, lesion-or transgene-induced, can seriously affect rotarod performance in adult mice; heavier mice typically demonstrate impairment on the task when compared with their lighter littermates. Unfortunately, there are few measures that can be taken to overcome this, other than to food restrict the heavier mice to produce comparable weights between the groups. However, if the underlying cause of the weight differences is not known, weight restriction may have unknown effects on the other physiological systems in the mice, for example energy metabolism that may also produce deleterious effects on performance.
4 The Footprint Test and Gait Analysis
4.1 Introduction
The analyses of the footfall patterns in mice can provide a highly sensitive functional readout of abnormality in the animals’ gait when running along a corridor or on a treadmill (16, 34–36). The test has been found to be sufficiently sensitive to distinguish between mouse background strains (31), and the longitudinal development of movement disorders in a number of neurological disease states including PD, HD, amyotrophic lateral sclerosis, cerebellar ataxia, and ataxia telangiectasia, plus SOD1 and complexin 1 knock-outs (16, 20, 23, 31, 34, 37, 38). It is also able to determine the effects of neurological lesions and spinal chord contusions on movement (39, 40). This section will focus on footprint analysis as measured by the manual footprint method rather than the digital tracking, treadmill systems, as the manual footprint method is accessible to all due to the minimal financial investment required, in contrast to the digital imaging systems, the cost of which can be prohibitive to some. Regardless of the approach, the assessments of gait are provided by measuring different aspects of the footfall pattern, for example stride length (39, 41), and fore-paw/hind-paw overlap, base width between the forepaws and hind-paws and movement time (16, 20). Digital systems are able to provide further measures (see below). These measures imbue the gait analysis tests with a high translational value, in that the motor output that is measured has direct correlates with measures in people. This coupled with the simplicity of the test, make the footprint test a user-friendly, informative, sensitive and inexpensive test of motor function with high translational value.
4.2 Materials and Methods
The footprint test in its simplest form requires nothing more than paint, paper and a corridor type of arena in which to test the animals. Typically, corridors are less than 1 m in length, but can be anything from a length of ∼10 cm (34), or greater. In our laboratory until recently, we use a 60 cm apparatus (31), which allows multiple strides to be assessed thereby providing greater reliability in the scoring (Fig. 3a–b). Typically, corridors are 10 cm in width, but again there is some variation. In some instances corridors are not used at all, with freely moving mice being filmed from below through a clear flooring (35, 42), however collecting consistent footfall patterns with this method may be difficult as the mice are frequently changing direction. Some corridors are fitted with dark goal boxes at one end or a tunnel to provide the mouse with an incentive to run from a lit area to a dark area where they feel secure.
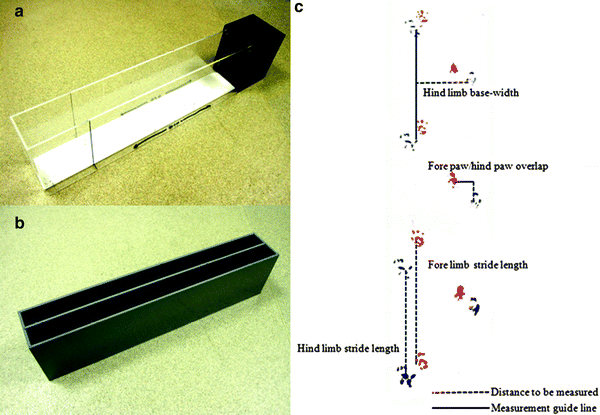

Fig. 3.
Runways for the gait analysis are simple corridors and can be constructed using various designs. The runway at the top (a) has the advantage of a goal box, but has the disadvantage of being wide (10 cm) which permits the mice to deviate on their paths resulting in increased variability in the footprint pattern. Having clear perspex sides may cause distraction. The lower runaway (b) is made of a dark plastic and has two 5 cm runways divided by a removable partition wall. The narrow construct maintains the consistent direction of the mouse. The three measures of gait (c) are stride length, base width and overlap, the measurement of which are depicted.
The mice should be habituated to the apparatus prior to training. During the habituation phase, the mice are permitted to explore the whole of the apparatus, including any goal boxes that may be present. Typically, two habituation sessions of 15 min each would be sufficient, but this may vary by mouse strain. Mice will usually run a corridor with little encouragement, but some mouse strains may prove to be more resistant, in which case a brief training regime may be required. Training consists of teaching the mice the location of the goal box and encouraging them to run the corridor to find it. Such training regimes tend to be rapid as the task is simple and consists of encouraging the mouse to move into the goal box, starting from a very short distance away and gradually increasing this distance until the mouse readily runs the full length of the corridor. If this is not successful, there are a number of other methods available that may aid the training procedure (see Notes below).
For the mouse to provide foot steps, the researcher is required to paint the fore and hind feet of the mice, typically in two different colours such that the fore and hind-paw prints are distinguishable. The paint to be used is the non-toxic, water-based paint that is readily available. The mouse then has to walk or run along a narrow corridor that has paper lining the floor, such that painted footprints are left as the mouse travels the length of the corridor. Once the mouse has run the corridor, the footfall patterns are analysed through the measurement of several parameters (Fig. 3c). Stride length is assessed by measuring the distance between the centre of the plantar of the fore foot, to the centre of the hind foot plantar on the same side of the body, within the same stride. Base width is the lateral (across the body of the mouse) measure of the distance between the front paws or the hind-paws and essentially measures the degree of lateral displacement of the limbs when the feet are planted. However, as the paw prints are produced in a moving animal, the corresponding front and hind limb prints are always out of alignment. The measurement of the base width distance is created by drawing a perpendicular line from the centre of one fore or hind limb across what would be the body of the animal, such that is bisects at a right angle, a second line from the plantar of the corresponding limb from the opposite side of the body. The base width is then the cross-body distance between the initial plantar and the bisection point. The final gait measurement is the degree of overlap between the fore and hind foot falls. When rodents walk in a straight line, the hind foot lands close to the same place as the fore foot of the same side of the body. Mice with motor deficits, commonly demonstrate a short fall in the overlap pattern. This measure may demonstrate a specific loss of function, for example rigidity, typically in the hind limbs. To measure overlap, the distance between the centre of the plantar of the front paw print and the corresponding hind footprint plantar of successive strides are measured. In addition to these measures, the mice are timed to run the corridor to provide a general measure of motor function. Ideally, at least two measures for each of the parameters should be taken.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


