CHAPTER 43 Neorickettsia risticii
ETIOLOGY
Neorickettsia risticii (formerly Ehrlichia risticii) is the etiologic agent of Potomac horse fever, also called “equine monocytic ehrlichiosis” or “equine ehrlichial colitis.” N. risticii has recently been classified based on genetic analysis in the genera Neorickettsia among three other species: Neorickettsia sennetsu (human agent of Sennetsu fever), Neorickettsia helminthoeca (agent of salmon poisoning in the dog), and an ehrlichia-like bacterium present in the metacercarial stage of the fluke Stellantchasmus falcatus (SF agent).1 Based on sequence analysis of the 16S ribosomal ribonucleic acid (rRNA) gene, N. risticii shares 98.9% homology to N. sennetsu, 94.8% to N. helminthoeca, and 99.1% to the SF agent. Strain variance has been determined among 11 N. risticii strains, with a maximum divergence of 0.7%.
EPIDEMIOLOGY
Potomac horse fever (PHF) was recognized originally in 1979 along the Potomac River in the state of Maryland.2 PHF is known to occur in 43 of the United States, three Canadian provinces (Nova Scotia, Ontario, Alberta), South America (Uruguay, Brazil), Europe (The Netherlands, France), and India. Isolation or detection of the causative agent from clinical cases of the disease using conventional cell culture or molecular detection by polymerase chain reaction (PCR) has only been reported from 13 states (California, Illinois, Indiana, Kentucky, Maryland, Michigan, New York, New Jersey, Ohio, Oregon, Pennsylvania, Texas, Virginia), Nova Scotia, Uruguay, and Brazil.
The epidemiology of N. risticii has been the subject of intensive research efforts for more than 20 years. The disease typically occurs near freshwater streams, rivers, and on irrigated pastures, mainly during middle to late summer (May to November). The seasonal incidence of the disease, the geographic distribution of PHF, and the experimental transmission by the intradermal route implied the involvement of a blood-sucking arthropod as a vector. The historical connection between other ehrlichial agents and tick vectors prompted many to regard ticks as prime candidates for the transmission of N. risticii. Therefore, many studies focused on identifying an arthropod vector for PHF. Despite intensive investigation, however, no evidence was found for spread of the disease by arthropod vectors such as ticks.3
The causative organism is present in the feces of experimentally infected horses and can be experimentally transmitted by the oral route using feces from infected horses. These findings, together with the close serologic and molecular relationship between N. risticii and N. helminthoeca isolated from flukes, suggest that the vector of N. risticii may not be an arthropod but instead a helminth closely associated with aquatic habitats. Barlough et al.4 provided strong evidence that trematodes, which use operculate freshwater snails as intermediate hosts, may be involved in the life cycle of N. risticii. This theory was confirmed when deoxyribonucleic acid (DNA) of N. risticii was detected by nested PCR in operculate snails (Pleuroceridae: Juga spp.) collected from stream waters in a northern California pasture where PHF is endemic. The results of sequencing PCR-amplified DNA from a suite of genes (16S rRNA, groESL heat shock operon, and 51-kDa major antigen genes) indicated that the source organism was clearly related to the type strain of N. risticii. The PCR-amplified product is associated with the presence of virgulate cercariae in the snail secretions5 (Fig. 43-1). The number of snails harboring the trematode stages varied from 3.3% to 93.3%, and the number of PCR-positive snails (3.3%-20%) appears to depend on the size of the snails, the month of collection, and geographic origin.
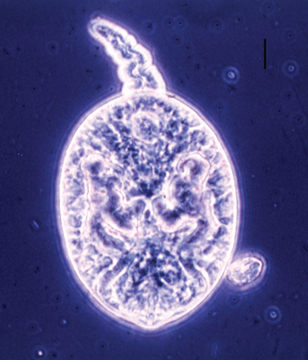
Fig. 43-1 Photomicrograph of virgulate cercaria released by pleurocerid snails of genus Juga (bar = 0.01 mm).
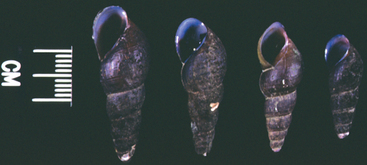
In northern California the species of snail incriminated in the life cycle of N. risticii is Juga yrekaensis, a common pleurocerid snail, which inhabits fresh or brackish stream water in the northwestern United States (Fig. 43-2). Additionally, DNA from N. risticii has been detected in virgulate cercariae in lymnaeid snails (Stagnicola spp.) from northern California, in virgulate xiphidiocercariae isolated from pleurocerid snails (Elimia livescens) in central Ohio, and from pleurocerid snails (Elimia virginica) in central Pennsylvania, suggesting that other types of snails may also harbor infected trematodes.5–7 This type of trematode is known to become encysted in the second intermediate host. N. risticii DNA has been detected by PCR in mesocercariae and metacercariae in various aquatic larval, nymphal, and adult insects such as caddisflies, mayflies, damselflies, and dragonflies in northern California and in central Pennsylvania7,8 (Fig. 43-3). PCR investigations suggest that the prevalence of aquatic insects harboring N. risticii varies from 10% to 80%.
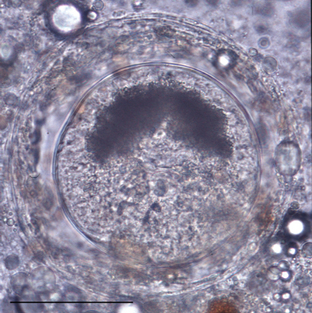
Fig. 43-3 Photomicrograph of metacercaria collected from caddisfly larva (bar = 0.2 mm).
(From Madigan JE, Pusterla N: Vet Clin North Am Equine Pract 16:487, 2000.)
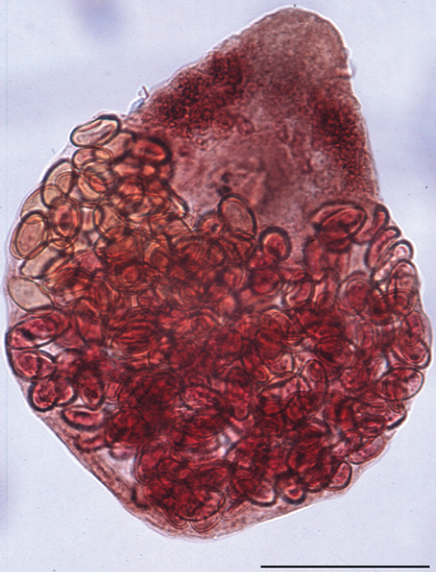
Recently, two potential helminth vectors, Acanthatrium spp. and Lecithodendrium spp., both infected with N. risticii, were found in the intestine of bats and birds collected in northern California and Pennsylvania9,10 (Fig. 43-4). These trematodes belong to the Lecithodendriidae family, common parasites of bats, birds, and amphibians in North America, which use pleurocerid freshwater snails as first intermediate hosts and aquatic insects as second intermediate hosts. Additional trematodes, members of the Lecithodendriidae or other families, may also act as vectors of N. risticii in other endemic regions of the United States.
Since N. risticii was first identified, no definitive reservoir host of the organism has been proposed. Seroepidemiologic studies have revealed the presence of antibody titers specific to N. risticii in domestic and wild animals such as dogs, cats, coyotes, pigs, and goats from regions in which PHF is endemic.11 A variety of nonequine mammalian species, such as mice, dogs, cats, and cattle, are susceptible to N. risticii.12–14 Based on vertical transmission of N. risticii in the trematode Acanthatrium oregonense and detection of N. risticii DNA in the blood, liver, or spleen of bats and swallows, it is speculated that these insectivores act as both definitive host of the helminth vector and natural reservoir of N. risticii.
The biologic activity of N. risticii in infected vectors has been recently investigated by the inoculation of PCR-positive trematode stages into horses and mice. Horses injected subcutaneously with N. risticii PCR-positive trematode stages (virgulate cercariae and sporocysts) collected from J. yrekaensis snails developed clinical signs and hematologic changes consistent with PHF.15 Furthermore, N. risticii was transmitted to mice using PCR-positive metacercariae isolated from caddisfly larvae (Dicosmoecus spp.).8 These data confirm that N. risticii is associated with a helminth vector and illustrate the value of PCR technology as a screening method for epidemiologic studies.
PATHOGENESIS
The mode of transmission of N. risticii has remained one of the greatest mysteries of PHF. N. risticii has been successfully transmitted by the intravenous, intramuscular, subcutaneous, intradermal, and oral routes using whole blood from naturally infected horses or with infected cell culture material.16–20 In light of recent epidemiologic discoveries concerning the vector of N. risticii and its helminth hosts, horses could conceivably be exposed to N. risticii through skin penetration by infected cercariae or by consuming infected cercariae in water or metacercariae in a second intermediate host such as an aquatic insect. One horse fed adult caddisflies (Dicosmoecus gilvipes) in northern California21 and two horses fed adult caddisflies (Cheumatopsyche campyla, Hydropsyche hageni) or a mixture of adult caddisflies and mayflies (Leucrocuta minerva) in central Pennsylvania developed PHF.7 These studies attempted to mimic the natural route of infection with N. risticii and showed that oral transmission using infected aquatic insects was not only possible, but also that the clinical disease produced was similar to that seen in naturally infected horses. Aquatic insects, such as caddisflies and mayflies, represent a likely source of infection because of their abundance in the natural environment, their high infection rate with N. risticii
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree