CHAPTER 62 Nematodes
Numerous nematode parasites infest and infect horses. The first section of this chapter describes the important nematodes of the gastrointestinal tract of horses. Nematode infections with clinical manifestations affecting the skin (onchocerciasis, habronemiasis) and the respiratory tract (lungworm infection) are discussed later in this chapter. Chapter 4 discusses nematode infection of neural and somatic tissues (Halicephalobus spp.); Chapter 10 discusses infection of ocular tissues (Thelazia spp.).
GASTROINTESTINAL NEMATODES
Craig Reinemeyer and Martin Krarup Nielsen
Strongylosis
The term strongylosis refers to infection with any of dozens of species of similar nematodes that reside as adults within the large intestine of equids. These parasites are classified in the family Strongylidae and are commonly known as “strongylid nematodes” or “strongyles.” Differences in morphology, biology, pathogenicity, anthelmintic susceptibility, and management strategies support the practicality of discussing the strongyles as two major groups: the subfamily Strongylinae (“large strongyles,” strongylins) and the subfamily Cyathostominae (“small strongyles,” cyathostomins, cyathostomes).1
Strongylinae (Large Strongyles, Strongylins)
Etiology.
All strongyles invade gut tissues after initial infection, but only larval stages of the genus Strongylus migrate to organs beyond the alimentary tract. Larvae of Strongylus vulgaris wander within the vascular system; those of Strongylus edentatus invade the liver and retroperitoneum; and Strongylus equinus larvae migrate within the liver and pancreas2 (see Pathogenesis). Because of their protracted migration, members of the genus Strongylus have long prepatent periods ranging from 5.5 to 12 months, depending on the species.
Epidemiology.
The role of the environment in the transmission of strongylosis is virtually identical for large and small strongyles. The cycle of transmission begins when strongylid eggs are passed in the feces of an infected host. Strongylid eggs are homogenous, and large strongyle eggs cannot be differentiated from those of cyathostomins. Equine feces usually contain adequate levels of moisture (>24%)3 and oxygen to support hatching and development of larval stages, and eggs are able to hatch when environmental temperatures exceed 8° C (46° F).4,5 The first larval stage (L1) hatches from an egg, feeds on organic material in the manure, molts to the second larval stage (L2), and eventually develops into an infective, third-stage larva (L3). Rates of egg hatching and larval development increase in direct proportion to environmental temperatures. Under field conditions, the minimum interval between egg passage and development to the infective stage is approximately 1 week.
Environmental conditions during spring and autumn are ideal for strongyle transmission in all regions. However, geoclimatic variations result in seasonal differences in strongyle transmission patterns during winter and summer. In northern temperate climates (above ∼40 degrees north latitude), summer is a favorable season for hatching of strongyle eggs and development into infective larvae.6 Northern winters are too cold for eggs to hatch, but larval survival is excellent. In contrast, southern temperate summers are too hot and dry for sustained larval persistence, but winter conditions are mild enough to allow some egg hatching as well as excellent survival of existing larvae.3,7 In terms of transmission potential, horses in southern temperate regions experience substantial risk during autumn through spring, whereas transmission in pastured, northern horses is virtually perennial.
In northern temperate climates, migrating Strongylus larvae return to the gut and begin egg production during spring.8 Third-stage larvae develop during spring and summer, and ingestion by grazing horses completes transmission. Adult large strongyles survive in the host through summer and autumn, become senescent during winter, and are replaced in the following spring by a new population.8 The annual epidemiology of Strongylus infection has not been studied in southern temperate regions.
Pathogenesis.
Large strongyles damage the host as both larval and adult stages. Within days of infection, L3 Strongylus vulgaris larvae invade the submucosa of the small intestine and molt to the L4 stage.9 These larvae enter local arterioles, burrow beneath the intima, and migrate proximally to the root of the anterior mesenteric artery (AMA).10 A proportion of S. vulgaris larvae continue to wander past this site, and migratory lesions have been observed in the root of the aorta and in other arterial branches.11 Larvae within the intima of the proximal AMA cause severe local arteritis, accompanied by focal enlargement, thrombi within the vessel lumen, and hypertrophy of the medial layer (Fig. 62-1). This lesion is classically described as a “verminous aneurysm,” although the arterial walls are neither thin nor dilated. Verminous arteritis is associated with an increased incidence of colic, although the pathophysiologic mechanisms are unknown. The simplest hypothesis is that emboli from the thrombus are carried distally and occlude the arterial supply of the gut. However, a necropsy survey of ischemic bowel lesions of horses failed to demonstrate emboli in the majority of cases.12 Other hypotheses for inducing colic include primary changes in gut motility13 and interference with local neurologic control.14
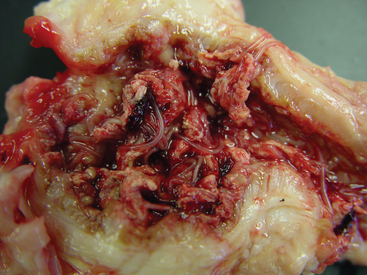
Fig. 62-1 Verminous arteritis caused by migrating larvae of Strongylus vulgaris.
(Courtesy Dr. Charles Faulkner, Department of Comparative Medicine, University of Tennessee College of Veterinary Medicine, Knoxville.)
Migrating larvae of Strongylus edentatus and S. equinus have been associated with hemorrhagic and inflammatory lesions in the liver, pancreas, and retroperitoneal tissues. These lesions do not cause distinct clinical signs but may contribute to the general inflammatory component of strongylosis. In the final stage of migration, all Strongylus larvae return to the large intestine and form nodules within the gut wall.15 Adult large strongyles emerge from these nodules to take up residence within the gut. The parasitic nodules can be greater than 1 cm in diameter and often are filled with purulent material whether occupied or vacant.
Adult large strongyles can use their buccal capsules to attach firmly to the mucosa of the cecum or ventral colon. These worms are said to “plug feed,” drawing small amounts of gut mucosa into the oral cavity and presumably ingesting tissue proteins. Rupture of local vessels may cause focal hemorrhage, but the consequences of adult feeding activities are only a minor component of strongylosis. One noteworthy species of large strongyle, Triodontophorus tenuicollis, feeds in colonies of 20 or more adults and causes large (1-3 cm), deep ulcers in the dorsal colon.2
Clinical Findings.
Strongylosis is characterized by nonspecific signs of weight loss and poor growth, rough hair coat, and compromised performance.16 Experimental infections caused pyrexia, tachycardia, anorexia, diarrhea, and listlessness.17 Larval S. vulgaris infections do not cause distinct clinical signs but are associated with an increased incidence of colic.
Diagnosis.
Infection with adult strongyles is easily demonstrated by using a variety of concentration techniques to detect strongylid eggs in the feces (see Chapter 58). Concentration techniques include simple fecal flotation using saturated salt or sugar solutions, as well as quantitative procedures such as the McMaster’s, modified Wisconsin, or modified Stoll technique.18 Regardless of methodology, the presence of strongyle eggs in the feces of grazing horses is ubiquitous, so the diagnostic value of fecal examination is not robust. Even quantitative techniques that calculate the numbers of eggs per gram of feces have limited interpretive value because strongylid egg counts are not correlated to the numbers of parasites that produced them, and they are not predictive of the severity of clinical parasitism.19,20
Strongylid eggs are homogenous, and those of cyathostomins cannot be differentiated from large strongyle eggs (Fig. 62-2). In mixed infections, more than 95% of strongyle eggs passed in the feces are produced by cyathostomins. Proportional contributions of various strongylid genera or subfamilies to the total egg count can be determined by fecal culture, which requires that feces containing strongyle eggs be kept moist, aerated, and incubated at room temperature for 10 to 14 days. The L3 larvae that develop in these conditions are collected and examined, and the proportional contributions by cyathostomins (Fig. 62-3) and the various genera of large strongyles (Fig. 62-4) can be calculated.18
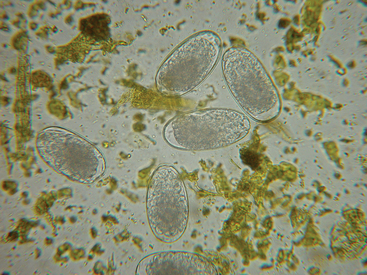
Fig. 62-2 Photomicrograph of typical strongylid eggs passed by large strongyles and cyathostomins.
(Courtesy Dr. Charles Faulkner, Department of Comparative Medicine, University of Tennessee College of Veterinary Medicine, Knoxville.)
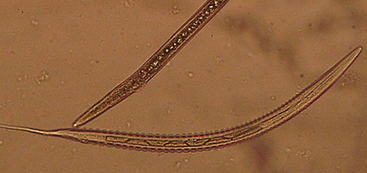
Fig. 62-3 Third-stage larva (L3) of cyathostomin nematodes.
(Courtesy Dr. Martin Krarup Nielsen, Department of Large Animal Sciences, Royal Veterinary and Agricultural University, Frederiksberg, Denmark.)
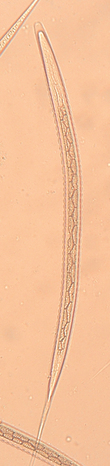
Fig. 62-4 Third-stage larva (L3) of Strongylus vulgaris, a large strongyle.
(Courtesy Dr. Martin Krarup Nielsen, Department of Large Animal Sciences, Royal Veterinary and Agricultural University, Frederiksberg, Denmark.)
In the 1990s, the ribosomal deoxyribonucleic acid (rDNA) from several species of large and small strongyles was sequenced and cloned.21 This allowed the first and second internal transcribed spacers (ITS-1 and ITS-2) to be used for species-specific amplification in polymerase chain reactions (PCR).22 This technology holds potential for the development of PCR-based diagnostic assays to detect and quantify the presence of large strongyle eggs in fecal samples. Such assays are currently under development, but no commercial tests are available at present.
Hematology and serum chemistry of horses with strongylosis often indicate mild anemia, leukocytosis with eosinophilia, hypoalbuminemia, beta-globulinemia, and elevations of IgG(T).15,23 Essentially all abnormalities are consequences of inflammation and protein-losing enteropathy.
Pathologic Findings.
Discrete hemorrhages, approximately 1 cm in diameter and occurring beneath the serosa of the ileum, have been termed hemomelasma ilei and are considered to be pathognomonic for recent S. vulgaris infection.11 The pathologic lesions caused by fourth-stage and fifth-stage S. vulgaris larvae within blood vessels have been characterized by scanning electron microscopy.24 Typical lesions consist of large thrombi surrounding larvae located within vessels with dilated lumens and thickened walls. Active coagulation is evident on the surfaces of larvae, thrombi, and vascular endothelium, where aggregations of red blood cells, platelets, and fibrin can be observed. The concurrent existence of a mature thrombus with additional, active thrombi indicates cumulative formation over time. The arterial walls become thickened by fibrosis and occasionally by dystrophic calcification.15 After thrombotic lesions are fully developed, the artery may never return to normal.25
On rare occasions, migrating Strongylus larvae have been associated with cerebrospinal disorders.15 More common are eosinophilic lesions in the pancreas26 or eosinophilic granulomas in the liver and epicardium.27
Therapy.
Several anthelmintics belonging to three chemical families are effective for treatment of adult large strongyles, but migrating larvae are susceptible to only three anthelmintic regimens (Table 62-1). Because of the inflammatory nature of lesions associated with migrating larvae, specific chemotherapy cannot be expected to result in immediate alleviation of clinical signs of strongylosis.
Prevention.
General approaches to strongyle control are discussed in greater detail in the following section on Cyathostominae. Chemotherapeutic approaches to control large strongyles include daily administration of pyrantel tartrate (2.64 mg/kg), which kills ingested strongylid larvae before they can invade gut tissues and establish infection.28 Unlike some populations of cyathostomins, resistance to pyrantel tartrate has not been demonstrated in large strongyles to date.
Eradication of large strongyles from a herd not only is feasible, but has been accomplished at a majority of well-managed horse farms in North America.29 Because larvicidal anthelmintics (see Table 62-1) can kill all stages of Strongylus larvae within the host, at least 6 months would be required after treatment before another adult population could develop and begin to contaminate pasture with eggs. By repeating larvicidal treatments at intervals of no greater than 6 months, new infections could not arise on a farm where all animals had been maintained on this program. Also, because the maximum duration of survival of free-living stages in the environment is approximately 1 year, no infective large-strongyle larvae could remain on pastures where this program had been maintained for 18 months or longer.30
Cyathostominae (Cyathostomes, Cyathostomins, Small Strongyles, Trichonemes)
Etiology.
The Cyathostominae found in North America belong to eight genera: Cyathostomum, Cylicocyclus, Cylicodontophorus, Cylicostephanus, Coronocyclus, Parapoteriostomum, Poteriostomum, and Petrovinema.31 Another genus, Gyalocephalus, has been assigned to a different taxonomic group, but it behaves biologically as a cyathostomin. In contrast to large strongyles, cyathostomins have shallow or cylindrical buccal capsules with which they attach weakly to the mucosa of the large intestine. Most small strongyle species are moderately sized nematodes, less than 1.5 cm in length by about 1 mm in diameter.
After ingestion by a grazing horse, L3 cyathostomins invade the mucosa of the cecum, ventral colon, or to a minor extent, the dorsal colon.32 Within 1 to 2 weeks after infection, a fibrous capsule develops around the larva,33 which is now said to be “encysted.” Small-strongyle larvae develop through multiple, sequential stages. The invading stage (early third-stage larva, or EL3) grows into a late third-stage larva (LL3), then molts into an early fourth-stage larva (EL4) and ultimately a late fourth-stage larva (LL4). All larval growth and maturation occur within the confines of a mucosal or submucosal cyst. The LL4 stage emerges from the cyst into the lumen of the large intestine and develops into a reproductive, adult nematode. Larval development may be progressive, producing an adult worm in as quickly as 5 to 6 weeks, or larvae may arrest development in the EL3 stage,34 and not undergo further maturation for up to 2.5 years.35
Epidemiology.
The bionomics of cyathostomin development and persistence in the environment are virtually identical to those of large strongyles, discussed previously. An annual cycle has been hypothesized for cyathostomin populations within the host.23 A large proportion of encysted larvae emerge from gut tissues during late winter or early spring and mature into adult cyathostomins. The reproductive activity of this new population is manifested by the spring increase in fecal egg counts.36 This population continues to reproduce through the summer months, when conditions are favorable for egg hatching and larval development. In northern climates, a second generation of adult cyathostomins may arise in late summer as a direct product of infection earlier in the grazing season. The majority of larvae ingested during autumn are destined to undergo arrested development within the host. This population overwinters within host tissues and emerges in the following spring to initiate another annual cycle.
Arrested development is a strategy employed by many nematode species to avoid unfavorable environmental conditions. Cyathostomin larvae specifically interrupt their development at the EL3 stage and can remain encysted in tissues for 2 years or longer.34,35 Small strongyle populations in northern climates undergo arrested development during winter, presumably to avoid environmental conditions that are unfavorable for egg hatching. Similarly, cyathostomin populations in the south would arrest during summer to evade hot, dry weather, which favors neither larval development nor persistence.
Pathogenesis.
Cyathostomin third-stage larvae cause mucosal inflammation of the cecum and ventral colon when they first invade the gut after ingestion.37 Within a few weeks, these larvae become surrounded by a fibrous capsule within the mucosa or submucosa of the host organ.33 The capsule protects the horse from the parasitic products inside the cyst, but it also shields the nematode from inflammatory and immune responses of the host. Minimal inflammation is observed around encysted larvae as long as the capsule remains intact (Fig. 62-5). Rupture of the cysts during larval emergence releases excretory and secretory products that have accumulated over time. Larval excystment results in lesions of focal edema, congestion, and hemorrhage, with accompanying leakage of tissue fluids into the bowel lumen. The cumulative severity of the lesions and their effect on the host are correlated to the numbers of larvae emerging. Adult small strongyles are ineffective plug feeders and cause minimal damage as mature parasites.38
Clinical Findings.
Strongylosis is characterized by weight loss and poor growth, rough hair coat, and compromised performance.16 Cyathostomins can cause these general clinical signs in the absence of a large-strongyle component. One specific syndrome, larval cyathostominosis, is a seasonal condition caused by synchronous emergence of large numbers of encysted cyathostomins.23 This syndrome is characterized by severe diarrhea, rapid weight loss, marked hypoproteinemia, and passage of numerous larval cyathostomins in the feces. In northern climates, larval cyathostominosis occurs most frequently in late winter, when encysted larvae are expected to emerge from arrested development. In southern regions, larval cyathostominosis is most common in late summer and autumn for the same reasons.39
Diagnosis.
Recent attempts to develop serologic assays to detect infection with encysted larval cyathostomins have shown great promise.40,41,42 Such assays would be of great value in equine practice because these stages cannot be detected by fecal examination, yet clearly are the most pathogenic. In addition, a PCR-ELISA (enzyme-linked immunosorbent assay) has been validated that can identify six different species of cyathostomins by fecal analysis.43,44 This technology allows parasitologists to study the relative impact of specific species in natural infections, as well as to identify species with higher levels of anthelmintic resistance.
Pathologic Findings.
Soon after larval ingestion, L3 cyathostomins penetrate the basement membrane of the epithelial cells of the tubular glands in the large intestine, where they provoke a fibroblastic reaction. This increases as the larvae grow, eventually leading to goblet cell hyperplasia and hypertrophy and distortion of the glands. Modest infiltration with lymphocytes and (in some cases) eosinophils occurs below and around the encapsulated larvae.37
The major lesions of cyathostomin infection occur during larval emergence and include typhlitis and colitis, which contribute to protein-losing enteropathy.45 Intense, focal eosinophilia may develop when the cyst wall is breached by an emerging larva.37
Therapy.
Adult cyathostomins are susceptible to several anthelmintics belonging to four chemical families, and two anthelmintic regimens demonstrate activity against encysted larvae (see Table 62-1). The efficacy of a dewormer against adult cyathostomins can be evaluated simply with a fecal egg count reduction (FECR) test (Box 62-1).
Most equine anthelmintics are labeled only for removal of adult and larval cyathostomins from the lumen of the gut; larvae encysted in the gut wall may not be affected. When mature worms are killed by deworming, larval stages emerge from tissues at varying intervals thereafter, apparently to reestablish reproducing populations. Anthelmintic treatment purportedly can even induce parasitic disease by promoting the emergence of arrested cyathostomin larvae from tissue cysts.47 In addition to the endogenous supplementation of adult populations, horses grazing on infective pastures rapidly acquire a new generation of larvae to maintain the cycle.
Prevention.
Strongyle infection is ubiquitous in grazing venues, so the major objective of control is to limit pasture contamination with feces containing strongylid eggs. This can be accomplished by targeting the general fecal component. In fact, the use of commercial vacuum units to remove manure from pastures at biweekly intervals was very effective for controlling the transmission of strongyles in the United Kingdom.48 However, pasture hygiene is labor intensive and requires expensive machinery and relatively flat paddocks for effective implementation. For grazing horses, pasture rotation has not been a successful control strategy because it is extremely complicated and requires extensive monitoring beyond the capacity of laypersons.
Rather than limiting fecal contamination, most strongyle control programs attempt to reduce the numbers of parasite eggs contained in the feces. This requires chemical intervention, so anthelmintics are the keystone of almost all strongyle control programs for horses. Administering pyrantel tartrate (2.64 mg/kg) daily in the feed is a preventive, chemical approach because it kills ingested strongylid larvae before they can invade gut tissues and establish infection. One concern about this program is that perennial use could interfere with the development of acquired immunity, especially by younger horses. In addition, populations of cyathostomins can develop resistance to pyrantel tartrate, rendering its daily use ineffective.49
A common strategy is to administer anthelmintics at a frequency that removes reproducing adults but also limits cumulative egg production by the herd over the course of a grazing season. Egg counts should decrease by more than 90% after the successful removal of a small-strongyle population. Ultimately, however, adult populations are replaced, reproduction resumes, and egg counts return to previous levels. The interval between effective treatment and resumption of significant egg production is known as the egg reappearance period (ERP).50 ERPs are quite predictable for various classes of equine anthelmintics. Benzimidazole or pyrantel pamoate treatments typically provide an ERP of 4 weeks, ivermectin prevents contamination for 6 to 8 weeks after treatment, and the ERP of moxidectin is approximately 12 weeks. By administering subsequent anthelmintic treatments at an interval determined by the ERP of the previous drug class, replacement worm populations are killed before they achieve full reproductive potential. Thus the quantities of eggs produced are limited, and pasture contamination is minimized. Fewer eggs yield fewer larvae, with a lowered risk of future infection for horses grazing that pasture.
Unfortunately, suppressive treatment tends to select for anthelmintic resistance, or at least for populations that reproduce faster and have shorter ERPs. Cyathostomin populations that are resistant to benzimidazole anthelmintics were first identified in the 1960s and are now widespread in most countries. Resistance to pyrantel salts is found primarily in North America, where it was documented on approximately 40% of horse farms in one survey.49 The lower prevalence of pyrantel resistance in the rest of the world is possibly explained by the exclusive availability of daily pyrantel tartrate in North America. The macrocyclic lactones ivermectin and moxidectin remain the only anthelmintics that are fully effective against equine strongyles. Consequently, these drugs are the most widely used in equine establishments. The potential evolution of macrocyclic lactone resistance by cyathostomins has been discussed extensively.51,52,53 Because no new classes of equine anthelmintics are anticipated in the near future, current control strategies must be revised to preserve the efficacy of available drugs for as long as possible.
Biologic control with nematode-trapping fungi has potential as a nonchemical approach to equine parasite control.54 These fungi are natural predators of nematodes and kill free-living larvae by trapping them in a network of hyphae. Fungal spores can be fed to animals so that they pass though the alimentary tract and inoculate the feces. Fungi trap nematode larvae as they develop in the feces and significantly reduce the numbers of larvae on pasture. At present, fungal products are not commercially available.
Modern approaches to cyathostomin control emphasize the use of effective drugs, high levels of parasite surveillance, and decreased intensity of treatment within the herd. These approaches can maintain health, limit pasture contamination, and decrease selection pressures for anthelmintic resistance. One such approach, termed selective treatment, is based on characterizing the contaminative potential of each horse in a herd, then designing control programs based on individual characteristics.55–58 The magnitude of strongylid egg counts of horses after a long sojourn from deworming can be used to classify individual animals as low, moderate, or high contaminators. Horses that are “low contaminators” apparently control parasitism on their own and do not contribute significantly to overall contamination of the premises. Such animals do not need to be dewormed at all if only cyathostomins are present on the premises. If large strongyles are a potential threat, low contaminators only need to be dewormed twice annually with a larvicide, at the beginning and end of a grazing season, to maintain eradication of large strongyles. Horses that are “high contaminators” should be the major focus of control and probably need repeated anthelmintic treatment throughout the grazing season. Finally, those horses that are “moderate contaminators” can be dewormed on a schedule that is intermediate between the two extremes. Eradication of cyathostomins is not a realistic goal for any control program.
Parascarosis
Etiology and Epidemiology
Parascaris equorum (ascarids, roundworms), as with other members of the superfamily Ascaridoidea, resides as an adult in the small intestine of its host. P. equorum is the largest nematode that infects horses, growing to approximately the size of a pencil when mature (Fig. 62-6). Thick-walled eggs are passed in the feces 10 to 15 weeks after infection59–61 (Fig. 62-7). Development of eggs to the infective stage in the environment requires about 10 days at optimal temperatures (25°-35° C [77°-95° F])62 and probably several weeks in cool conditions. The infective stage is an egg containing a second-stage larva (L2).2 Larvated eggs are ingested from the environment, hatch in the small intestine, and the liberated L2 ascarids enter the portal circulation and are carried to the liver. Larvae are migrating through the liver within 24 hours of infection.60 After approximately 1 week, larvae are carried to the lungs, where they break out of the vasculature and enter alveoli. These larvae migrate up the airways or are coughed up into the pharynx, where they are swallowed and return to the small intestine to complete maturation.
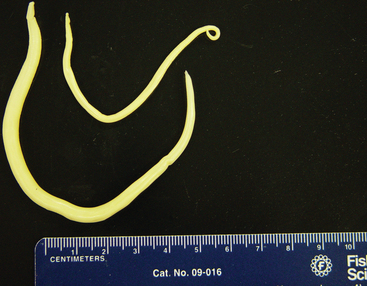
Fig. 62-6 Parascaris equorum adults; male (top) and female (bottom).
(Courtesy Dr. Charles Faulkner, Department of Comparative Medicine, University of Tennessee College of Veterinary Medicine, Knoxville.)
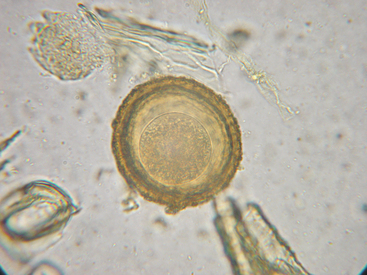
Fig. 62-7 Egg of Parascaris equorum.
(Courtesy Dr. Charles Faulkner, Department of Comparative Medicine, University of Tennessee College of Veterinary Medicine, Knoxville.)
The major features of the epidemiology of parascarosis are the extreme persistence of the infective stage in the environment and the predominance of infection in juvenile horses. Larvated Parascaris eggs remain viable for 10 years or more, so one patent infection on a premise can affect several future generations of foals. Susceptible animals acquire infection by ingesting larvated eggs from the environment. Unlike strongylids, ascarid infections can be transmitted in confinement venues as well as on pasture. Parascaris eggs possess a sticky, protein coating63 that allows them to adhere to a variety of surfaces, including vertical walls and the hair coat or udder of a mare. Parascaris infection is found almost exclusively in juvenile horses less than 18 months of age.64 Horses develop extremely effective acquired immunity against Parascaris, and it is unusual to see patent infections in mature horses. Infections in juvenile horses often involve hundreds of worms, comprising a liter or more of parasites. Weanlings and yearlings ultimately lose their ascarid infections, probably through a combination of acquired immunity and senescence of the adult worm population. Parascaris
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree